FDA Commissioner Resigns Unexpectedly
/By Pat Anson, PNN Editor
The head of the U.S. Food and Drug Administration resigned unexpectedly Tuesday, just days after a critical report on 60 Minutes that alleged the FDA “opened the floodgates” to the opioid crisis.
“I’m immensely grateful for the opportunity to help lead this wonderful agency,” FDA commissioner Scott Gottlieb, MD, tweeted. ”This has been a wonderful journey and parting is very hard.”
In a lengthier statement to FDA staff, Gottlieb cited family reasons for his departure.
“There’s perhaps nothing that could pull me away from this role other than the challenge of being apart from my family for these past two years and missing my wife and three young children,” Gottlieb said, indicating he would remain on the job until next month.
Gottlieb is a 46-year old cancer survivor and a former consultant to several drug companies. He commutes to Washington DC from his home in Westbury, Connecticut, where he lives with his wife and three daughters.
President Trump tweeted that Gottlieb “has done an absolutely terrific job” and “he and his talents would be greatly missed!”
SCOTT GOTTLIEB, MD
There was no indication that Gottlieb was in trouble or that he was forced out. The FDA is currently involved in a number of complex and controversial regulatory issues, from high drug prices and e-cigarettes to medical marijuana and the opioid crisis.
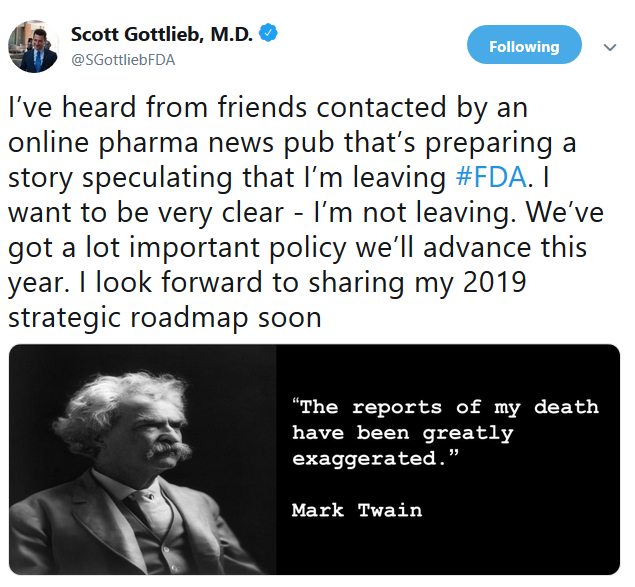
The timing of Gottlieb’s departure is puzzling, however, because he tweeted two months ago that he had no intention of resigning after hearing from friends about speculation in the news media that he was leaving.
“I want to be very clear — I’m not leaving. We’ve got a lot of important policy we’ll advance this year,” Gottlieb tweeted, adding a famous quote from Mark Twain that reports of his death “have been greatly exaggerated.”
The 60 Minutes report alleged that the FDA caved into lobbying pressure from the pharmaceutical industry in 2001 by changing the warning labels on OxyContin and other opioid medications to indicate they were effective for long term use.
Gottlieb was not working at FDA when the agency made its labeling decision, but pledged last week in a lengthy essay that the FDA would “learn from past mistakes” and take “a much more aggressive approach to regulatory action” involving opioids.
Gottlieb joins a long list of agency directors and cabinet members who have resigned from the Trump administration, including former Health and Human Services Secretary Tom Price, who was forced out over excessive travel expenses and other ethical lapses, and former CDC director Brenda Fitzgerald, who resigned after it was disclosed she invested in tobacco and drug companies.