Evidence Lacking for Spinal Cord Stimulators
/By Drs. Ian Harris, Adrian Traeger and Caitlin Jones
In an episode of the Australian Broadcasting Corporation’s “Four Corners” this week, the use of spinal cord stimulators for chronic back pain was brought into question.
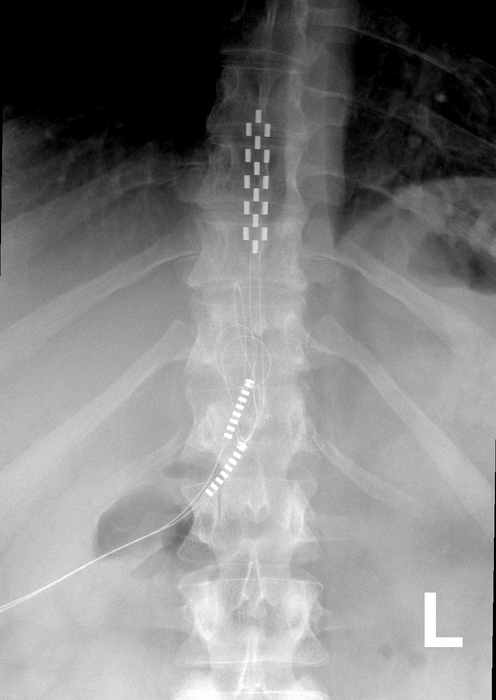
Spinal cord stimulators are devices implanted surgically which deliver electric impulses directly to the spinal cord. They’ve been used to treat people with chronic pain since the 1960s.
Their design has changed significantly over time. Early models required an external generator and invasive surgery to implant them. Current devices are fully implantable, rechargeable and can deliver a variety of electrical signals.
However, despite their long history, rigorous experimental research to test the effectiveness of spinal cord stimulators has only been conducted this century. The findings don’t support their use for treating chronic pain. In fact, data points to a significant risk of harm.
People with chronic pain are suffering infections, shocks and even more pain from a device advertised as safe and effective. There are now calls for them to be recalled following a Four Corners investigation. https://t.co/qb1i0A47Lv
— ABC News (@abcnews) April 9, 2024
What Does the Evidence Say?
One of the first studies used to support the effectiveness of spinal cord stimulators was published in 2005. This study looked at patients who didn’t get relief from initial spinal surgery and compared implantation of a spinal cord stimulator to a repeat of the spinal surgery.
Although it found spinal cord stimulation was the more effective intervention for chronic back pain, the fact this study compared the device to something that had already failed once is an obvious limitation.
Later studies provided more useful evidence. They compared spinal cord stimulation to non-surgical treatments or placebo devices (for example, deactivated spinal cord stimulators).
A 2023 Cochrane review of the published comparative studies found nearly all studies were restricted to short-term outcomes (weeks). And while some studies appeared to show better pain relief with active spinal cord stimulation, the benefits were small, and the evidence was uncertain.
Only one high-quality study compared spinal cord stimulation to placebo up to six months, and it showed no benefit. The review concluded the data doesn’t support the use of spinal cord stimulation for people with back pain.
The experimental studies often had small numbers of participants, making any estimate of the harms of spinal cord stimulation difficult. So we need to look to other sources.
A review of adverse events reported to Australia’s Therapeutic Goods Administration found the harms can be serious. Of the 520 events reported between 2012 and 2019, 79% were considered “severe” and 13% were “life threatening”
.We don’t know exactly how many spinal cord stimulators were implanted during this period, however this surgery is done reasonably widely in Australia, particularly in the private and workers compensation sectors. In 2023, health insurance data showed more than 1,300 spinal cord stimulator procedures were carried out around the country.
In the review, around half the reported harms were due to a malfunction of the device itself (for example, fracture of the electrical lead, or the lead moved to the wrong spot in the body). The other half involved declines in people’s health such as unexplained increased pain, infection, and tears in the lining around the spinal cord.
More than 80% of the harms required at least one surgery to correct the problem. The same study reported four out of every ten spinal cord stimulators implanted were being removed.
The cost here is considerable, with the devices alone costing tens of thousands of dollars. Adding associated hospital and medical costs, the total cost for a single procedure averages more than $A50,000 (US$ 32,542). With many patients undergoing multiple repeat procedures, it’s not unusual for costs to be measured in hundreds of thousands of dollars.
Rebates from Medicare, private health funds and other insurance schemes may go towards this total, along with out-of-pocket contributions.
Insurers are uncertain of the effectiveness of spinal cord stimulators, but because their implantation is listed on the Medicare Benefits Schedule and the devices are approved for reimbursement by the government, insurers are forced to fund their use.
Industry Influence
If the evidence suggests no sustained benefit over placebo, the harms are significant and the cost is high, why are spinal cord stimulators being used so commonly in Australia? In New Zealand, for example, the devices are rarely used.
Doctors who implant spinal cord stimulators in Australia are well remunerated and funding arrangements are different in New Zealand. But the main reason behind the lack of use in New Zealand is because pain specialists there are not convinced of their effectiveness.
In Australia and elsewhere, the use of spinal cord stimulators is heavily promoted by the pain specialists who implant them, and the device manufacturers, often in unison. The tactics used by the spinal cord stimulator device industry to protect profits have been compared to tactics used by the tobacco industry.
A 2023 paper describes these tactics which include flooding the scientific literature with industry-funded research, undermining unfavourable independent research, and attacking the credibility of those who raise concerns about the devices.
More than three million Australians are living with chronic pain, and it's become a breeding ground for exploitation.
— ABC News (@abcnews) April 8, 2024
Watch Four Corners: Pain Factory, now on ABC iview.#4Cornershttps://t.co/cZ9Qrt0Lsk
Many who suffer from chronic pain may feel disillusioned after watching the Four Corners’ “Pain Factory” report. But it’s not all bad news. Australia happens to be home to some of the world’s top back pain researchers who are working on safe, effective therapies.
New approaches such as sensorimotor retraining, which includes reassurance and encouragement to increase patients’ activity levels, cognitive functional therapy, which targets unhelpful pain-related thinking and behaviour, and old approaches such as exercise, have recently shown benefits in robust clinical research.
If we were to remove funding for expensive, harmful and ineffective treatments, more funding could be directed towards effective ones.
Ian Harris, MD, is a Professor of Orthopedic Surgery at University of New South Wales. He is a practicing orthopedic trauma surgeon and directs a research unit specializing in surgical outcomes and the evidence base for surgery.
Adrian Traeger, PhD, is a Research Fellow at the Institute for Musculoskeletal Health at The University of Sydney. He is a physiotherapist who specializes in treating low back pain and other musculoskeletal conditions.
Caitlin Jones, PhD, is a postdoctoral research associate at the University of Sydney. Her research evaluates the benefits and harms of treatments for musculoskeletal conditions.
This article originally appeared in The Conversation and is republished with permission.