New Migraine Prevention Drugs Making Inroads
/By Pat Anson, PNN Editor
Over half the patients taking a new class of medication designed to prevent migraines say the benefits of treatment outweigh the drugs’ side effects, according to a new survey.
The drugs prevent migraines by blocking a protein — calcitonin gene-related peptides (CGRP) -- from binding to nerve receptors in the brain. Since 2018, the FDA has approved three injectable CGRP inhibitors for migraine prevention and recently approved the first oral tablet for migraine treatment.
Although the drugs are still relatively new, a recent survey of over 4,700 migraine patients by Health Union found that about a third (29%) are currently using a CGRP medication, while 12% had used one in the past.
Most said the drugs were effective at migraine prevention and worth the side effects, which include constipation, fatigue and weight gain. Only 9 percent said the drugs were not worth the side effects.
“CGRPs are in many ways a treatment revolution,” says Brian Green, vice-president of community development for Health Union. “There has not been a new class of medication specifically designed for treatment of migraine for decades. So this really is groundbreaking.”
Green said patients with chronic severe migraine are more likely to be early adopters of CGRP therapy, as opposed to people who have episodic migraines and fewer attacks.
Patients on CGRP therapy were more likely to say their migraine attacks increased over time and that they experience a wide array of symptoms, including head pain, brain fog, difficulty concentrating, fatigue, loss of words, memory loss and sensitivity to touch.
“Their symptoms are so severe they want the first available new treatment,” Green told PNN.
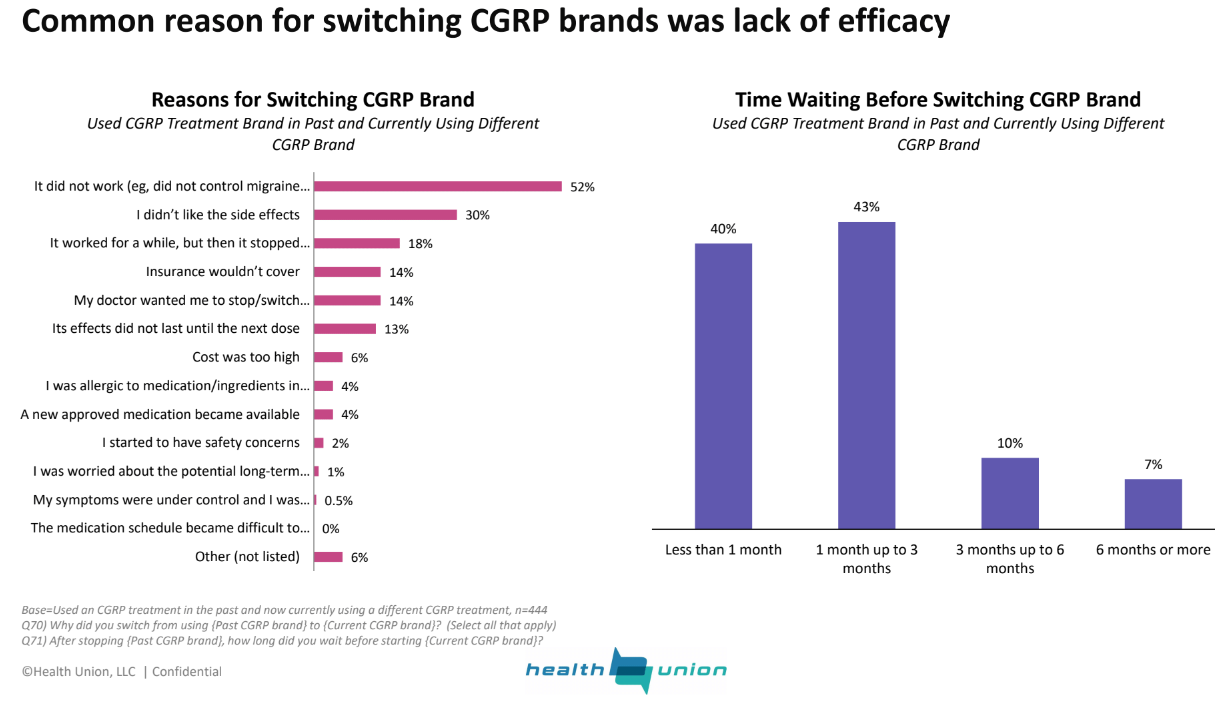
But the early adopters were also more impatient. Health Union’s survey found that patients who were not satisfied with a CGRP inhibitor wasted little time switching to a new brand. About 40% waited less than a month and 43% waited up to 3 months. Most of those who switched said the drugs did not work or stopped working after an initial period of efficacy.
The three CGRP inhibitors currently on the market are Aimovig, Ajovy, and Emgality, which are taken by injection about once a month. The first oral CGRP for migraine treatment, Ubrelvy, was approved by the FDA last month and is expected to be available in the next few weeks.
Most patients surveyed by Health Union said they would prefer taking a daily CGRP pill as opposed to a monthly injection.
Constipation was the leading side effect reported by patients getting CGRP injections. Nearly a third said the drugs made them constipated, while others complained of reaction at the injection site (16%), fatigue (15%), weight gain (12%) and dry mouth (11%).
About half the patients surveyed said they were still using a triptan or over-the-counter pain medication for migraine relief. Antidepressants and Topamax were the most commonly used medications for migraine prevention.
Regardless of the drugs used, only 12% of patients said their migraines were well controlled under their current treatment plan.