By Michelle Wyrick
For more than a decade, the United States has been running a vast, uncontrolled policy experiment in medical care. Under the banner of “opioid reduction” and “overdose prevention,” regulators have steadily restricted, stigmatized, and in many cases effectively eliminated access to stable, physician-supervised treatment for pain, anxiety, and other chronic disabling conditions.
The results of this experiment are now visible everywhere, and they are not subtle. Patients are sicker, more desperate, more marginalized, and more exposed to dangerous unregulated substances than at any point in modern medical history.
This outcome should not surprise anyone. It is not an accident. It is the predictable result of applying prohibition logic to medicine.
When legitimate patients are cut off from stable, supervised, pharmaceutical-grade treatment, they do not stop having pain. They do not stop having anxiety, severe depression, neurological disease, connective tissue disorders, autoimmune conditions, or the many other illnesses that produce chronic suffering.
They look for substitutes. And there will always be substitutes.
This is not a moral statement. It is a basic fact of human biology and behavior.
Demand for relief from suffering is not eliminated by supply restrictions. It is merely displaced into less safe, less predictable, and less medically supervised channels.
This dynamic is not unique to opioids. It is a universal feature of prohibition systems. Alcohol prohibition in the early 20th century did not end drinking. It drove production into unregulated, often toxic forms and empowered criminal supply chains. Modern drug prohibition has not eliminated drug use. Instead, it has ensured that the drugs people do use are increasingly potent, adulterated, and dangerous.
The same pattern is now playing out inside medicine itself.
For decades, physicians used opioid analgesics, benzodiazepines, and other controlled medications in a personalized, risk-benefit framework. This was not perfect medicine, but it was recognizable medicine. Doctors assessed individual patients, monitored them, adjusted doses, and discontinued treatment when risks outweighed benefits. The vast majority of stable patients used these medications without chaos, without dose escalation, and without the kinds of outcomes now routinely attributed to the “opioid crisis.”
Beginning in the mid-2010s, this model was replaced with something very different. Guidelines were transformed into rigid limits. Clinical judgment was replaced by fear of regulators. Medical boards, insurers, pharmacies, and hospital systems began enforcing population-level dose ceilings and forced tapering policies that took little or no account of individual patient physiology, genetics, or clinical history.
This shift was justified using public health language, but it was not actually evidence-based medicine. It was administrative medicine.
The core assumption behind this approach was simple and deeply flawed. If you reduce access to prescription opioids, you reduce addiction and overdose.
In the real world, the opposite happened.
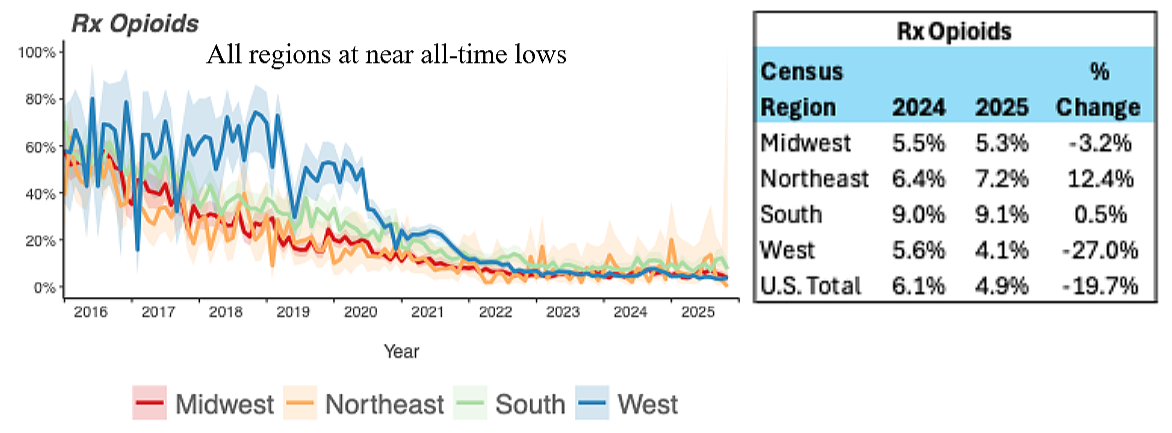
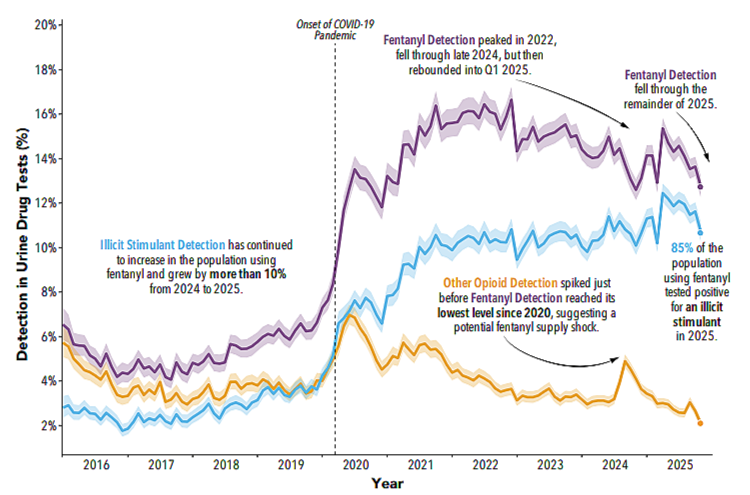
As prescription access fell, overdose deaths rose. Not slowly. Not ambiguously. They rose sharply and continuously, driven almost entirely by illicit synthetic opioids such as fentanyl and its analogues. This is not a coincidence. It is substitution.
When patients and non-patients alike lose access to regulated, dosed, known substances, the market does not disappear. It mutates. It becomes more concentrated, more dangerous, and more lethal.
From a pharmacological standpoint, this is exactly what one would predict. When supply is restricted, traffickers move to higher potency products that are easier to transport and conceal. This is why fentanyl replaced heroin, and why heroin replaced opium, and why alcohol prohibition favored spirits over beer. The same pressure operates everywhere prohibition is applied.
In medicine, this has produced a grotesque paradox. The very policies sold as “harm reduction” have forced more people into the most dangerous drug environment in history.
But the harm does not stop with overdose statistics.
For millions of legitimate patients, the new regime has meant something quieter but equally devastating. Forced tapers. Sudden discontinuations. Blacklisting by pharmacies. Doctors who will not treat pain at all. Clinics that advertise only “non-opioid” care, regardless of diagnosis, severity, or prior response.
These patients are often described in policy discussions as if they were abstractions. In reality, they are people with connective tissue disorders, spinal injuries, advanced arthritis, neuropathies, autoimmune diseases, post-surgical damage, and complex multi-system conditions. Many were stable for years or decades. Many were functional. Many worked, raised families, and lived ordinary lives.
When their treatment is removed, they do not return to some baseline healthy state. They collapse.
Some become housebound. Some lose the ability to work. Some develop severe depression and suicidality. Some are driven, reluctantly and fearfully, to seek relief outside the medical system.
This is the part of the story that is still not being honestly confronted.
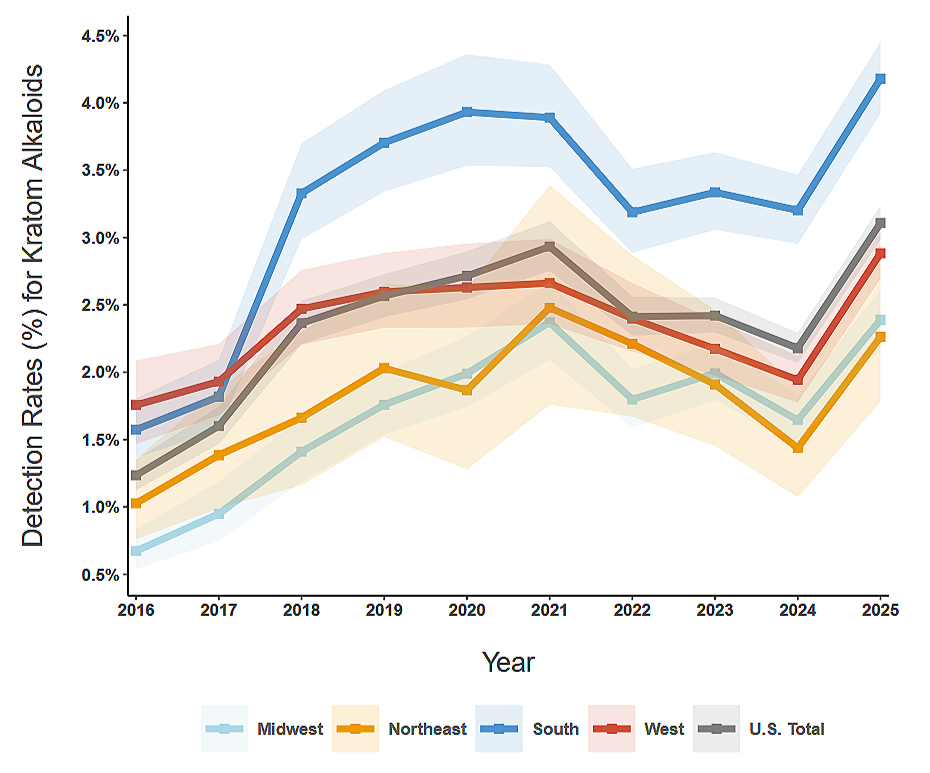
People do not seek unregulated substances because they want to. They seek them because the medical system has left them with no humane alternative.
This is not “addiction” in the simplistic, moralized sense that is often implied. It is survival behavior in the context of untreated suffering.
From a systems perspective, the current policy framework violates one of the most basic principles of risk management. If you remove a safer, regulated option while demand remains constant, you do not eliminate risk. You increase it.
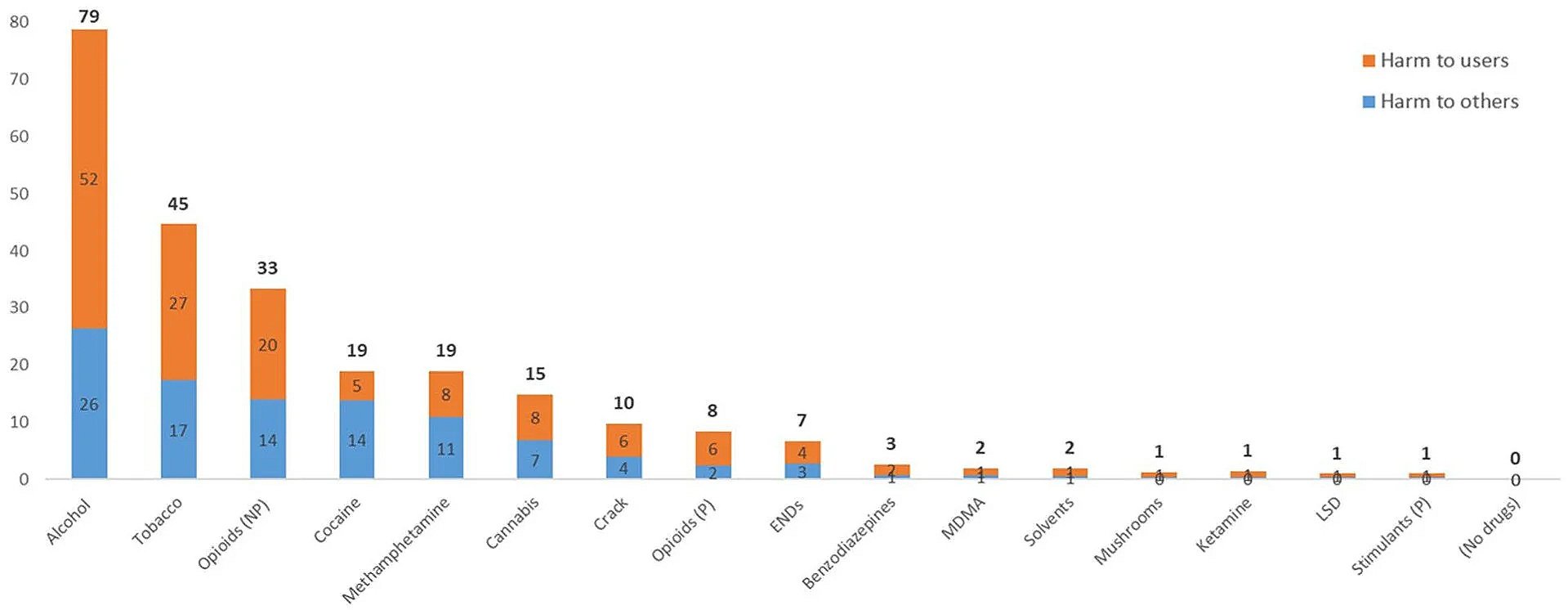
Pharmaceutical-grade medications have known dosages, known purity, known pharmacokinetics, and some degree of medical oversight. Gray and black market substances do not. They vary wildly in potency. They are often contaminated. They are frequently misrepresented. The margin for error is small, and the consequences of error are fatal.
This is why the shift from prescription opioids to illicit fentanyl has been so deadly. It is not because fentanyl is uniquely evil. It is because unregulated supply chains, extreme potency, and unpredictable dosing is a perfect storm.
A rational harm-reduction strategy would aim to pull people into safer, supervised, medically controlled channels. Instead, current policy does the opposite.
It pushes people out.
There is also a deeper scientific problem with the one-size-fits-all approach that now dominates pain and psychiatric care. Human beings do not respond to drugs uniformly. Genetics, metabolism, receptor expression, enzyme function, comorbid conditions, and prior exposure all profoundly shape both benefit and risk. Pharmacogenetics has made this increasingly obvious, yet policy continues to pretend that a single dosage threshold can define safety for everyone.
This is not medicine. It is bureaucratic simplification masquerading as science.
Some patients tolerate and benefit from opioid therapy at doses that would be excessive for others. Some cannot tolerate even low doses. Some respond better to one class of medication than another. The same is true for benzodiazepines, antidepressants, stimulants, and nearly every drug class in existence.
The proper response to this variability is individualized care, not blanket restriction.
Instead, clinicians are now taught, implicitly and explicitly, that avoiding regulatory risk matters more than relieving suffering. The result is widespread medical abandonment.
From an ethical standpoint, this should be alarming. Medicine is supposed to be organized around the care of the patient in front of the clinician, not the appeasement of distant agencies.
From a public health standpoint, it is also failing by its own stated metrics. Overdose deaths continue. Illicit markets continue to grow. Patients continue to be driven out of care.
This is not because the problem is unsolvable. It is because the framing is wrong.
We are not dealing with a battle between “medicine” and “drugs.” We are dealing with a battle between regulated, supervised, accountable systems and unregulated, chaotic, lethal ones.
History has already shown us how this ends. Every time.
Prohibition logic has never worked in any domain. Not alcohol. Not drugs. Not sex work. Not abortion. Not gambling. It does not eliminate demand. It ensures that demand is met in more dangerous ways.
If policymakers actually cared about safety and harm reduction, they would reverse course.
They would restore rational, individualized medical prescribing. They would protect clinicians who practice careful, documented, patient-centered care. They would stop forcing stable patients into destabilizing tapers. They would bring people back into the healthcare system instead of pushing them into gray and black markets.
They would also start telling the truth about what has happened.
The current crisis is not the result of doctors prescribing too compassionately. It is the result of a system that replaced medicine with fear, and then called the outcome “public health.”
We can continue down this path, and watch the death toll and human suffering rise year after year. Or we can admit what history, pharmacology, and basic systems theory already tell us.
You cannot ban your way to safety.
You can only regulate, supervise, and care your way there.
And right now, we are doing the opposite.
Michelle Wyrick is a Board Certified Psychiatric Registered Nurse and a Clinical Hypnotist in Gatlinburg, Tennessee.