Survey Finds Patients Wary of Trying CGRP Migraine Drugs
/By Pat Anson, PNN Editor
Khloe Kardashian and Serena Williams have their work cut out for them. Despite their endorsement of a new type of migraine medication, many patients remain wary of the drugs and few have tried them, according to a large new survey.
The annual survey of nearly 4,700 migraine patients by Health Union found that about one in four (26%) are currently using a preventive CGRP medication, down from 29% in last year’s survey. And only 11 percent of patients said they were using a CGRP to treat migraine pain.
CGRP stands for calcitonin gene-related peptides, a protein that binds to nerve receptors in the brain and triggers migraine pain. Since 2018, the FDA has approved four injectable CGRP inhibitors (Aimovig, Ajovy, Emgality, Vyepti) to prevent migraine and two CGRP tablets for acute migraine (Ubrelvy, Nurtec). The latter two were recently endorsed by reality star Kardashian and tennis star Williams.
Although Eli Lilly, Teva, Amgen, and other drug companies have aggressively marketed CGRP medications and even given the drugs away for free to get people to try them, sales growth has been slow. Only Aimovig and Emgality are used by at least 10 percent of migraine patients.
PERCENTAGE OF PATIENTS CURRENTLY USING CGRP INHIBITORS
Most migraine patients continue to rely on older and cheaper medications such as triptans, anti-depressants, anti-convulsants, over-the-counter drugs, and Botox injections.
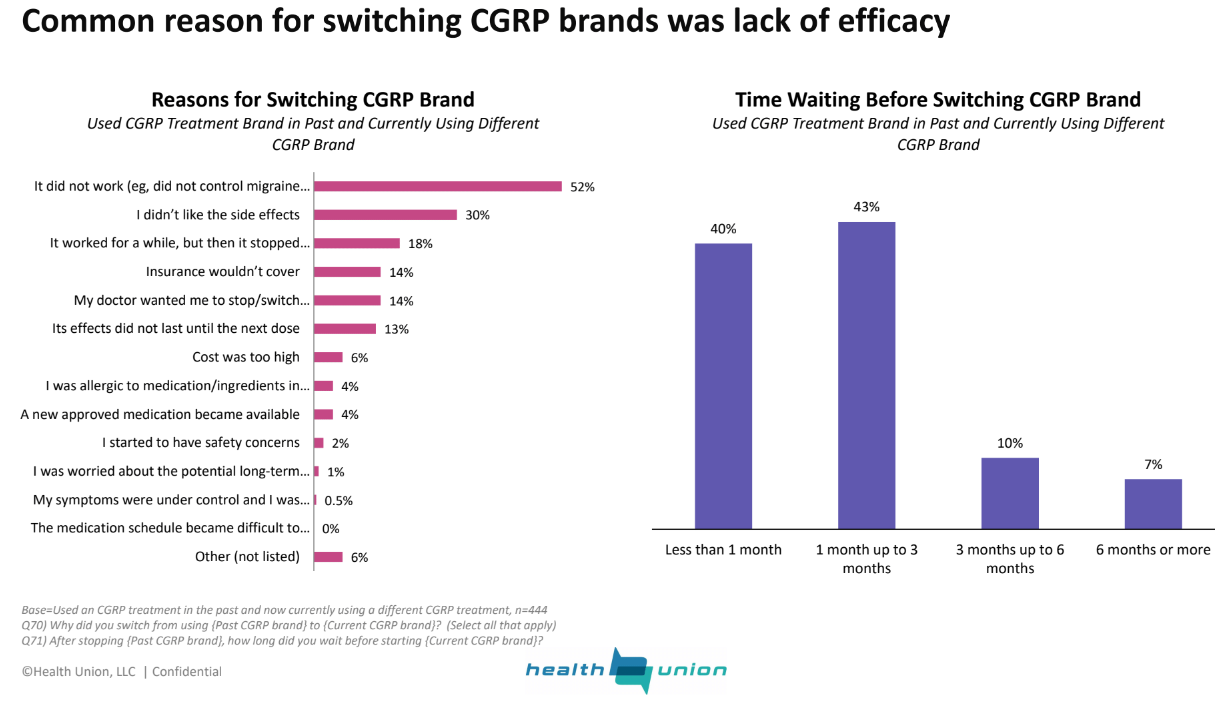
The Health Union survey helps explain why. While most patients are aware of CGRP inhibitors and nearly half (43%) had tried a preventive CGRP, most stopped taking them after trying just one brand. That’s not uncommon for migraine sufferers, who often have try multiple treatments before finding one that works.
“Everybody’s experience with treatment is different. And the fact that there are multiple brands available is actually a really good thing. Because some of them just happen to work better for some people than others,” says Brian Green, Health Union’s vice-president of community business solutions.
CGRP’s do work for some patients. The Health Union survey found that 58% of patients currently using a preventive CGRP reported having less head pain. And nearly half said they didn’t react as strongly to migraine triggers such as loud noises and bright light.
Those who had heard of preventive CGRP medications but had not tried them cited a number of reasons:
44% Doctor has not recommended it
27% Concerned about side effects
21% Concerned about long-term safety
19% Can’t afford them
14% Insurance won’t cover
CGRP medications are not cheap. Eight doses of Nurtec, the acute CGRP endorsed by Kardashian, can cost over $1,000 without insurance.
Nearly half the patients surveyed said they were still using triptans or over-the-counter pain medication for migraine relief. Antidepressants and Topamax were the most commonly used medications for migraine prevention.
Migraine affects more than 37 million people in the United States, according to the American Migraine Foundation. In addition to headache pain, migraine can cause nausea, blurriness or visual disturbances, and sensitivity to light and sound. Women are three times more likely to suffer from migraines than men.