FDA Approves First Implant That Zaps Rheumatoid Arthritis
/By Madora Pennington
A nerve stimulation implant recently approved by the FDA reduces symptoms of rheumatoid arthritis by stimulating the vagus nerve with mild electronic pulses. It’s the first FDA-approved neuromodulation device for adults with moderate-to-severe rheumatoid arthritis (RA).
The vagus nerve stimulator (VNS), created by SetPoint Medical of Valencia, California, is the size of a coffee bean. It is implanted in the left side of the neck under anesthesia during an outpatient procedure.
The device delivers electronic pulses into the vagus nerve for just one minute each day to calm the immune system and have an anti-inflammatory effect. RA causes the immune system to attack the lining of joints, damaging cartilage and eroding bone.
“The approval of the SetPoint System, the first-in-class neuroimmune modulation platform, represents a transformative milestone in the management of autoimmune diseases,” Murthy Simhambhatla, PhD, CEO of SetPoint Medical, said in a press release. “We plan to introduce the SetPoint System in targeted U.S. cities this year, followed by expansion across the country starting in early 2026.”
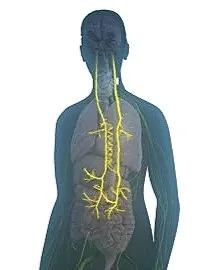
In auto-immune diseases like RA, the function of the vagus nerve becomes impaired. The nerve starts in the brainstem and runs through the neck, chest and abdomen, where it branches out into many organs.
The vagus nerve helps regulate the nervous system, including heart rate, blood pressure, respiration and digestion. When the nerve malfunctions, it causes inflammation and disrupts the immune system.
In a clinical trial of 242 patients, just over half (51.5%) had at least a 20% improvement in RA symptoms after 24 weeks with the SetPoint implant. Most patients tolerated the device well, but two experienced side effects of vocal cord weakness and hoarseness.
SETPOINT MEDICAL IMAGE
The SetPoint System is intended for RA patients who have not benefited from medications designed to calm the immune system, known as Disease-Modifying Antirheumatic Drugs (DMARDS) or the much stronger biologics that target specific immune cells. Doctors can monitor the device with an iPad application and patients can recharge it themselves with a wireless charger.
Vagus nerve stimulation is not new. In 1997, the FDA approved VNS for epilepsy. The implanted device is installed in the chest like a pacemaker to reduce seizures or even stop ones in progress.
VNS can also treat chronic pain, by helping the brain "turn down" pain signals and reduce pain indirectly through its anti-inflammatory effects.
Other types of pain for which VNS has demonstrated effectiveness are chronic migraine, chronic primary headache, cluster headache, fibromyalgia, chronic pancreatitis, esophageal pain, irritable bowel syndrome, neuropathic pain, musculoskeletal pain, osteoarthritis, and chronic low back pain.
SetPoint is investigating whether its technology can also treat multiple sclerosis, Crohn's disease and other autoimmune conditions.