Flexible Implant Could Be Alternative to Spinal Cord Stimulators
/By Pat Anson
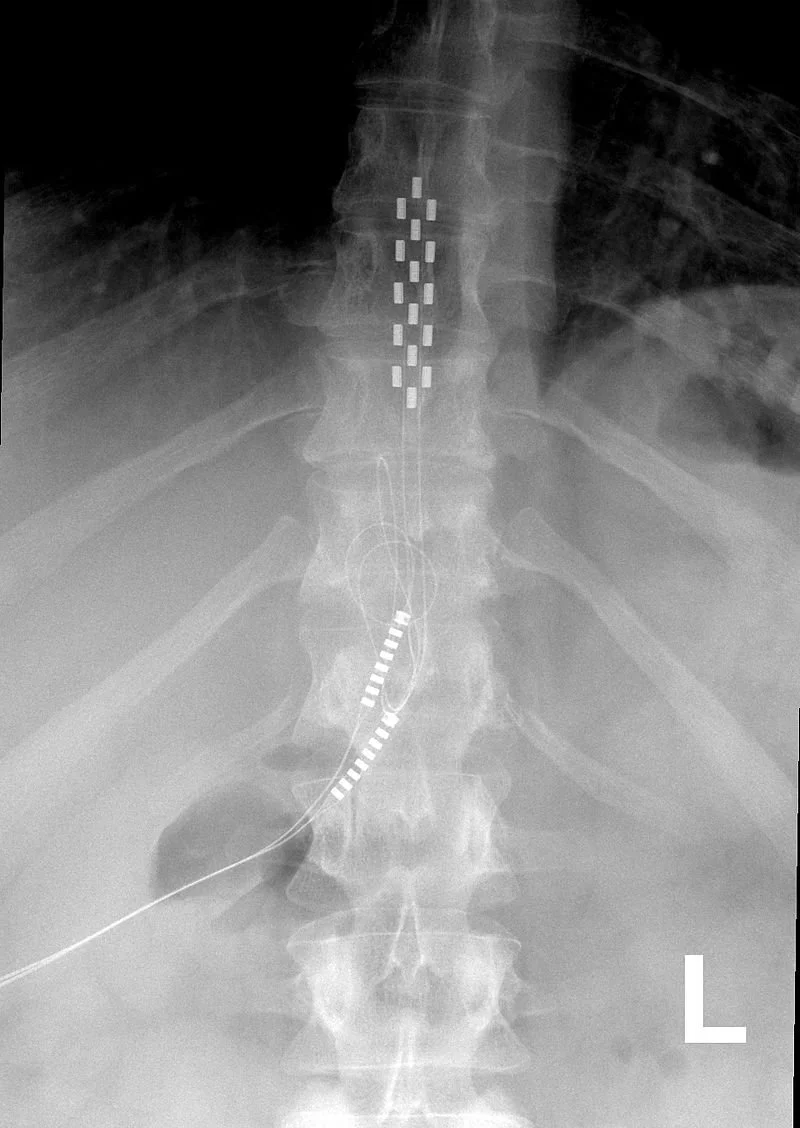
Spinal cord stimulators have long been fraught with problems. First developed in the 1960’s, the devices are surgically implanted near the spine to deliver mild electric impulses that block pain signals from traveling to the brain.
Although their design and technology have improved over the years, making them smaller, rechargeable and programmable, spinal cord stimulators (SCSs) still have high failure rates and poor safety records. Recent studies have also questioned their effectiveness in treating back pain.
Researchers at the University of Southern California have developed an alternative: a small, flexible, wireless, and battery-free implant that could overcome some of the limitations of SCSs.
NATURE ELECTRONICS
The experimental device, recently introduced in a paper published in Nature Electronics, is powered by a wearable ultrasound transmitter and uses machine learning algorithms to customize treatment for each patient. The implant is designed to bend and twist, unlike traditional stimulators that tend to be bulky and are hard-wired to batteries.
“Implantable percutaneous electrical stimulators… are expensive, can cause damage during surgery and often rely on a battery power supply that must be periodically replaced. Here we report an integrated, flexible ultrasound-induced wireless implantable stimulator combined with a pain detection and management system for personalized chronic pain management," wrote a team of researchers at USC’s Alfred E. Mann Department of Biomedical Engineering.
The researchers tested the wireless stimulator in laboratory rodents experiencing different levels of pain. They found the device accurately predicted the levels of stress experienced by the animals and adapted the electrical stimulation it delivered, easing most of their pain.
“We classify pain stimuli from brain recordings by developing a machine learning model and program the acoustic energy from the ultrasound transmitter and, therefore, the intensity of electrical stimulation," researchers said. "The implant can generate targeted, self-adaptive and quantitative electrical stimulations to the spinal cord according to the classified pain levels for chronic pain management in free-moving animal models."
The research team is planning further tests on other lab animals, with the goal of eventually testing the device in clinical trials on humans.
About 50,000 traditional spinal cord stimulators are implanted every year in the U.S., where they are promoted as alternatives to opioids and other pain treatments. However, a 2022 study found that SCSs do not reduce patient use of opioids, epidurals, steroid injections or radiofrequency ablation. About a fifth of patients experienced complications so severe the devices had to be removed or revised.