Medicare Pilot Program Will Use AI To Decide If Pain Treatments Are Worth the Cost
/By Pat Anson
Medicare patients in six states who need epidural steroid injections, cervical fusions, spinal cord stimulators, arthroscopic knee surgery and other treatments for chronic pain will soon have their prior authorization requests reviewed by artificial intelligence (AI) to decide whether the treatments are worth the cost.
The Centers for Medicare & Medicaid Services (CMS) is launching a 6-year pilot program on January 1, 2026 called the Wasteful and Inappropriate Service Reduction Model --- known as “WISeR” for short.
WISeR will cover Original Medicare patients in New Jersey, Ohio, Oklahoma, Texas, Arizona, and Washington who seek treatment for chronic pain, impotence, incontinence, and burns or wounds needing skin and tissue substitutes.
WISeR will review over a dozen treatments that CMS considers low-value, potentially unsafe, or suspicious because of prior reports of fraud and wasteful spending. The low-value treatments alone cost Medicare nearly $6 billion in 2022.
“CMS is committed to crushing fraud, waste, and abuse, and the WISeR Model will help root out waste in Original Medicare,” CMS Administrator Dr. Mehmet Oz said in a press release. “Combining the speed of technology and the experienced clinicians, this new model helps bring Medicare into the 21st century by testing a streamlined prior authorization process, while protecting Medicare beneficiaries from being given unnecessary and often costly procedures.”
Under traditional or Original Medicare, most covered services do not require prior authorization, but Medicare Advantage (MA) plans often do. For that reason, CMS is partnering with private MA plans that have more experience using AI and other advanced technologies to process prior authorization requests. If a request is denied by WISeR, the agency says it will then be reviewed before a final decision “by licensed clinicians, not machines.”
CMS claims that WISeR will “expedite decision making” and not change coverage for traditional Medicare beneficiaries, who “retain the freedom to seek care from their provider or supplier of choice.”
Those providers, however, will be incentivized with higher Medicare payments if they participate in WISeR and show they can reduce the use of low value treatments and help lower Medicare spending.
Pain treatments that will be reviewed under the WISeR Model include these procedures:
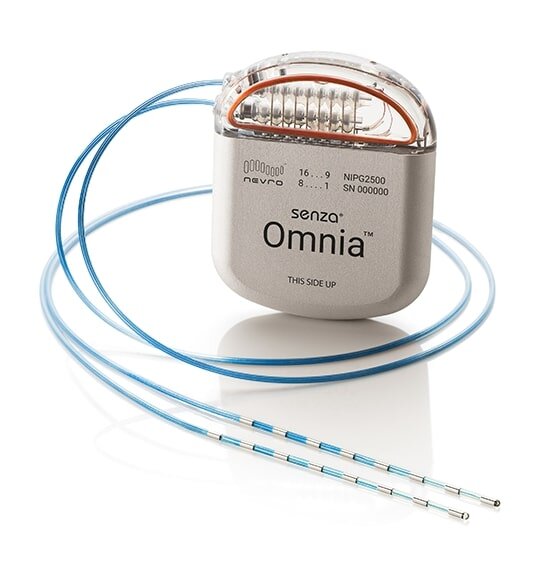
Electrical Nerve Stimulation
Deep Brain Stimulation for Essential Tremor and Parkinson's Disease
Vagus Nerve Stimulation
Surgical Removal or Ablation of Nerves
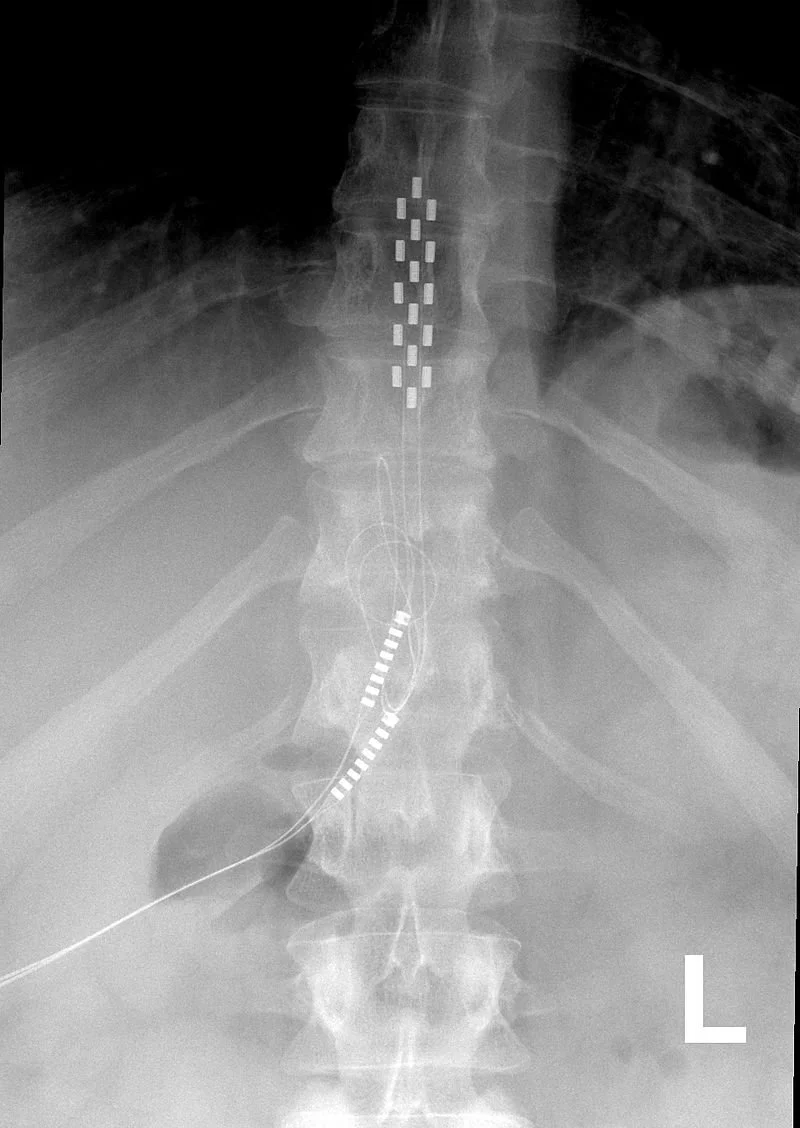
Epidural Steroid Injections (excluding facet joint injections)
Percutaneous Vertebral Augmentation (PVA) for Vertebral Compression Fractures
Cervical Fusions
Arthroscopic Surgery for Knee Osteoarthritis
Percutaneous Image-Guided Lumbar Decompression for Spinal Stenosis
Many of these treatments have already drawn scrutiny for being ineffective or costly. Studies have found that spinal cord stimulation, for example, has no benefit for back pain; while epidural steroid injections, nerve blocks and nerve ablation have been found to have little or no benefit.
‘Perverse Incentives’
Not surprisingly, the WISeR Model has drawn criticism from physicians who perform the procedures, who decry the use of AI and algorithms to make healthcare decisions.
“We firmly believe that (WISeR) will jeopardize patient access to care, create more administrative burdens for physicians, offer perverse payment incentives for third-party vendors, and represent a substantial reversal of progress toward this Administration’s goal of prioritizing patients over paperwork,” a coalition of 23 neurosurgeon organizations wrote in a letter to Dr. Oz.
“Decision criteria to be used by participating vendors — including algorithms, scoring models, and evidence-based guidelines — remain a “black box,” leaving stakeholders with little to no insight into how prior authorization determinations will be made.”
Patient rights groups and some politicians say WISeR will create new roadblocks for Medicare patients needing treatment.
“While prior authorization is often described as a cost-containment strategy, in practice it increases provider burden, takes time away from patients, limits patients’ access to life-saving care, and creates unnecessary administrative burden,” Rep. Ami Bera (D) and Rep. Suzan DelBene (D) said in a recent letter to Dr. Oz.
“The use of prior authorization in Medicare Advantage shows us that, in practice, WISeR will likely limit beneficiaries’ access to care, increase burden on our already overburdened health care work force, and create perverse incentives to put profit over patients.”
About 12% of prior authorization denials by Medicare Advantage insurers were appealed in 2023, and more than 80% of them were overturned, according to the Center for Medicare Advocacy.
An HHS Inspector General's report in 2018 found “widespread and persistent” problems involving denials of care by Medicare Advantage. Another report in 2022 found 13% of denied requests actually met Medicare’s rules and should have been approved.
“(WISeR) is) a backdoor way of putting everybody in a Medicare Advantage plan,” Carrie Graham, executive director of the Medicare Policy Initiative told Cleveland.com. “It’s a first step to getting rid of, or downgrading, the freedom that traditional Medicare provides.”