Microcurrent Therapy: The Healing Electrical Stimulation You’ve Never Heard Of
/By Madora Pennington
If you suffer from chronic muscle or soft tissue pain, a physical therapist or doctor may have recommended you get a TENS unit.
A traditional TENS (Transcutaneous Electrical Nerve Stimulation) device sends a low-voltage current through an injured area of the body, attempting to disrupt pain signals and stimulate endorphins, the body’s natural painkillers. The reviews of TENS are mixed. Some people experience relief from TENS, but many do not.
There is another kind of TENS that few patients and providers know about called microcurrent therapy (MCT). These devices deliver an electric current so small, the user might not feel anything. That’s because the current approximates the body’s own energy flows. The goal of MCT therapy is not to block pain sensation, but to encourage actual healing.
The human body itself is a complex electrochemical machine. Your cells generate low levels of electricity through chemical reactions called “biocurrents” -- which power bodily functions, regulate nerve signaling, boost cellular growth and energy, reduce inflammation, and so on. When body tissue is damaged, it produces an altered current that doesn’t work as well.
Stimulating the body with an external microcurrent accelerates tissue repair, wound healing, and muscle recovery. In short, it speeds healing by assisting with energy at the cellular level. The current from a traditional TENS, while low, is still much higher than the electrical currents the body runs on, so it does not improve the electrical functioning of cells.
For me, I never felt much benefit from a traditional TENS. So when I read articles about microcurrent therapy, I wanted to try it. I asked my physical therapist, and she had never heard of anything but a traditional TENS current, even though she is a recent graduate of a doctoral program, and an excellent PT.
I checked the various TENS units I already own, and none had the capacity to produce a microcurrent.
John Hubacher, President and CEO of Pantheon Research, a biomedical instrument manufacturing company, thinks microcurrent therapy may have gotten left behind because it was unclear why it worked so well. Without a clear mechanism for physiological action, it’s hard to get studies done. But now research is emerging that shows that MCT has the potential to change a cell's physiological processes.
Microcurrents have been shown to improve skin ulcers, varicose veins, and wound healing. It is also being used for cosmetic purposes as a “natural facelift” to tighten and tone skin, stimulate facial muscles, and boost collagen and elastin production. There are practically no side-effects, and it can be safely used in elderly populations.
Combining microcurrent therapy with exercise can be very helpful. Microcurrent used before and after a workout improves fat breakdown and reduces muscle soreness. Mice with atrophied muscles experienced muscle regrowth from the application of microcurrent therapy.
A 2025 paper remarked that this low-risk, powerfully therapeutic and inexpensive technology is grossly underutilized due to lack of awareness, even though studies support that it improves pain and function in many musculoskeletal conditions.
“Despite a growing body of evidence highlighting its therapeutic potential, MIC (MTC) therapy remains underutilized across many areas of medicine. Its subsensory, low-intensity electrical currents offers a non-invasive, pain-free alternative to traditional electrotherapies like TENS, without triggering muscle contraction or discomfort,” wrote lead author Sarahrose Jonik, MD, a Resident of Internal Medicine at Penn State College of Medicine.
“MIC therapy shows promise as an adjunctive modality capable of supporting tissue repair, reducing inflammation, and modulating pain, particularly in complex, chronic, or refractory conditions.” .
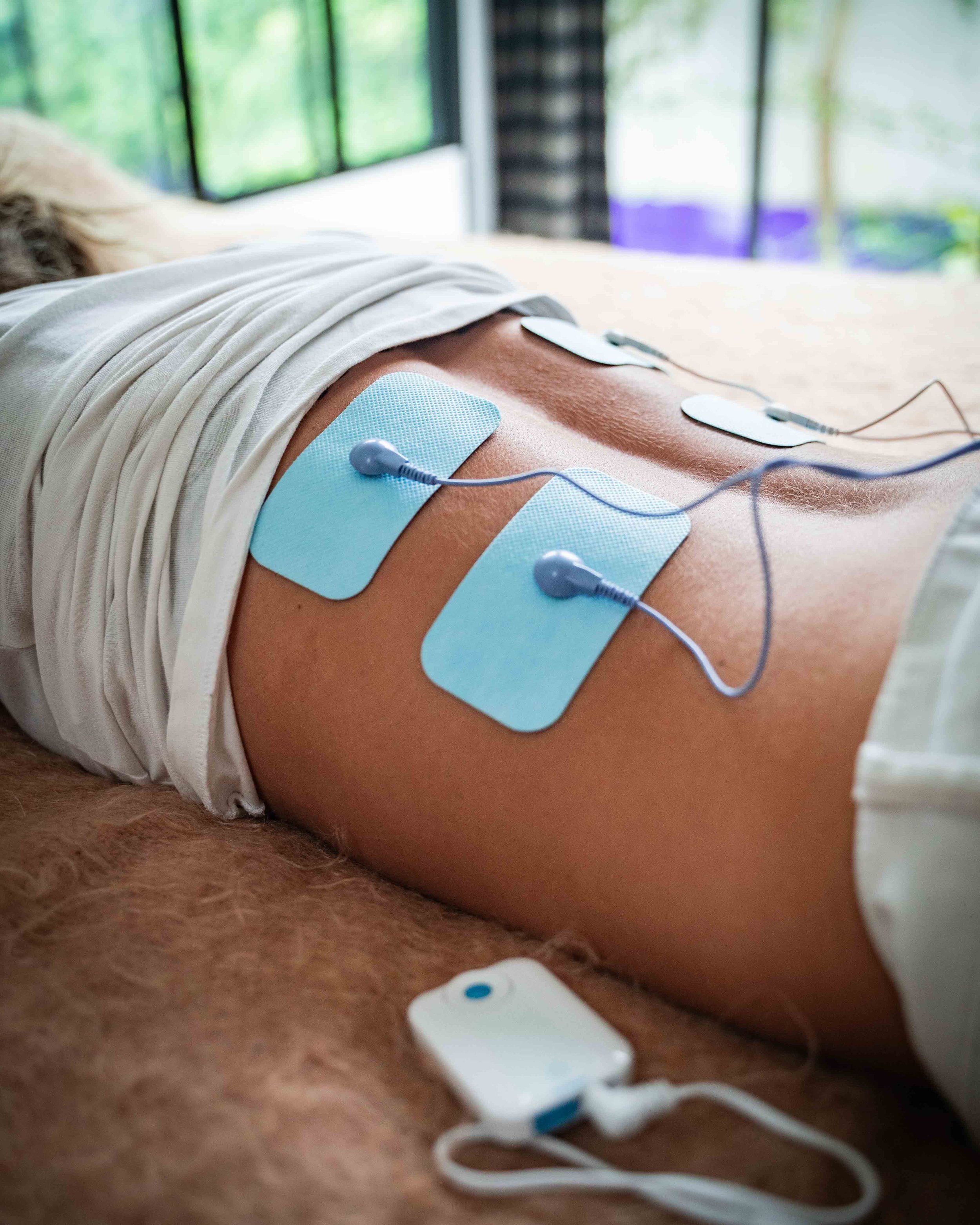
After an internet search, I bought the InTENSity 12 made by Compass Health. It looks and operates like a typical TENS and costs about the same, but produces a microcurrent. Like a TENS device, it has sticky pads that you attach to the skin near the area you want to work on.
I first used the InTENSity 12 on an area around my hip that is constantly tight. There, my muscles overwork to compensate for an old knee injury. It is an area I always have to work on and have had much physical therapy for.
The microcurrent absolutely melted the tension like no stretching, heat, massage, or trigger point release ever came close to doing. I did some stretching afterwards. The area released even more deeply and completely. My formerly hyperactive muscles stayed soft and easy to stretch for days.
Using the microcurrent TENS on other painful areas caused by other old injuries and a neuropathy flare, I felt relief that lasted for days. It left me wondering if this extra power, when delivered at the same level at which my cells operate, caused healing that my body was not doing on its own.