When Drug Tests Go Wrong
/By Pat Anson, Editor
Robin Haas was driving to Disneyworld with her husband and three children in 2008 when their vehicle was rear-ended by a truck on a Florida highway. The accident left Robin with chronic back pain, and she had 17 surgeries over the next 11 months to repair her damaged spine.
It was only the beginning of her problems.
Last year Robin was kicked out of a pain management practice after two office urine tests failed to find any trace of the fentanyl patch she was wearing for pain relief – a red flag for physicians that a patient may be diverting a drug.
Initially, Robin says her doctor didn’t seem too concerned.
“When it happened the second time, he said ‘Don’t worry about it. It’s happened with several of my patients with the fentanyl patches,’” Robin said
About a week later, she was shocked to get a certified letter from the doctor discharging her.
“I don’t know what happened. I really don’t,” Robin told Pain News Network. “I was just mortified. I never did anything wrong in pain management. Ever.”
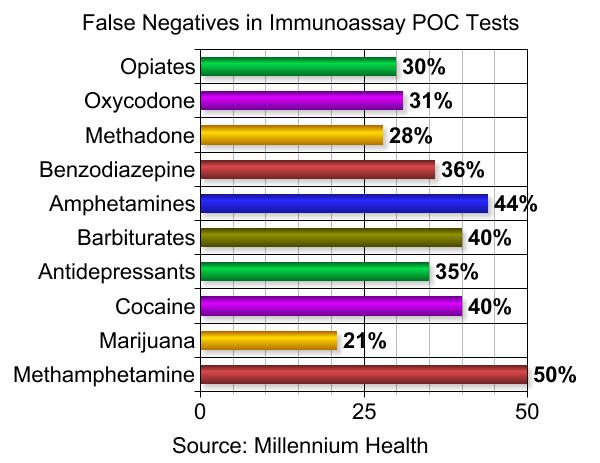
What happened to Robin is not uncommon. According to a recent study, immunoassay urine tests widely used by pain management doctors to screen patients for drug use are wrong about half the time – frequently giving false positive or false negative results.
“Clearly, people don’t know how to interpret these tests,” said Jeffrey Fudin, a pharmacist and patient advocate, who says most physicians have no idea how inaccurate immunoassay testing is.
“I’m positive that they don’t. I get probably 50 emails a week from all over the country from concerned physicians and nurse practitioners who want to make the right decision, but they’re not sure what to do,” said Fudin.
"The other problem is there are no standards. You can go to five different providers and be treated five different ways for the same results.”
Fudin says fentanyl may not show up on an opiate screen because it has a different chemical structure compared to most commonly prescribed opioids. There can also be false negatives because an opioid is simply prescribed in too low of a dose to be detected.
Some medications can also trigger false positives for an illicit drug. Widely used pain relievers like naproxen and ibuprofen, for example, can trigger a false positive for marijuana.
To help doctors correctly interpret immunoassay results, Fudin is developing an online app called Urintel that can help them decide whether to take a negative or positive drug screen seriously – and whether to order more reliable and more expensive confirmation testing in a laboratory.
“Basically, it’s educational and it’s not punitive to the patient,” said Fudin about his app.
“My goal is to make opioid therapy as safe as possible and to make it individualized for each patient. And also to be fair, not only to patients, but providers because it’s not their fault that they don’t have training in pharmacokinetics or biochemistry. It takes a lot of things to understand the complexity of this.”
Fudin’s app may be too late to help pain patients like Robin Haas. She just hopes more patients aren’t wrongly accused of diverting or abusing drugs because of a test that is so often wrong.
“I guarantee not one of my pills has ever hit the street,” says Robin.
The 43-year old Florida resident says the pain clinic told her that her urine samples were re-tested in a laboratory – at a cost of $18,000 – but the results came back the same. She’s still not sure what went wrong. The clinic has refused to identify the laboratory it used or provide her with the lab results. She had to find a new doctor.
“It’s a horrible thing to happen to people. And when you’re having to deal with chronic pain to begin with, nobody should have to go through it,” she says.
Have you been wrongly accused of failing a drug test?
Tell us your story. Send an email to editor@PainNewsNetwork.org.
We respect your privacy.