DEA Cuts Oxycodone Supply, But Raises Production of Morphine in Surprise Move
/By Pat Anson
The Drug Enforcement Administration is moving ahead with its plan to reduce the supply of oxycodone by over 6% in 2026, while at the same time significantly raising its production quota for morphine. There will be small reductions in the supply of hydrocodone, codeine and other Schedule II opioids this year.
The move to increase the supply of morphine by 10.5% is surprising, as the agency proposed cutting morphine production by over half a percent a little over a month ago.
The DEA officially announced its plans January 5 in the Federal Register, 35 days after a December 1 deadline set for the agency in the Controlled Substances Act (CSA). Under the CSA, the DEA has broad legal authority to set annual aggregate production quotas (APQs) for opioids and other controlled substances.
The December 1 deadline is important because it gives the pharmaceutical industry time to prepare for the coming year by adjusting drug manufacturing and distribution schedules. The DEA’s chronic failure to meet that deadline in previous years has contributed to shortages, according to drug makers.
Over 5,000 public comments were received by the DEA in response to the agency’s initial APQs for 2026. Most comments pleaded with the agency not to make any further cuts in the supply of opioids, many of which are already in short supply at pharmacies and hospitals.
“I oppose cutting production for controlled medications at this time as there is already a shortage for many of these medications and patients are often not able to obtain their prescriptions. Cutting production during a shortage will only exacerbate the problem and increase patient suffering,” Hannah Khalil wrote in a public comment echoed by many others.
The DEA, however, was dismissive of claims about opioid shortages, saying it was not responsible for them.
“Drug shortages may occur due to factors outside of DEA's control such as manufacturing and quality problems, processing delays, supply chain disruptions, or discontinuations,” the DEA said. “Currently, FDA has not listed on its Drug Shortage website any nationwide shortages of oxycodone and hydrocodone products.”
While it is true the FDA does not currently have oxycodone or hydrocodone on its shortage list, the American Society of Health-System Pharmacists (ASHP) has listed both opioids on its shortage list since 2023. Limited supplies of oxycodone and hydrocodone are available from some manufacturers, according to the ASHP, while others have the medications on back order.
The difference between the FDA and ASHP shortage lists is that the FDA relies on drug manufacturers to report shortages, while the ASHP proactively surveys both pharmacies and drug makers about their inventories. That arguably makes it superior to the FDA’s methodology.
Ironically, the DEA itself has challenged the reliability of the FDA’s drug shortage list.
“DEA has made it clear it does not trust FDA’s information, as it does not consider many of the shortages that FDA verifies to be legitimate,” the General Accountability Office (GAO) said in a 2015 audit report. “They do not believe FDA appropriately validates or investigates the shortages.”
Increased Morphine Production
The DEA offered no explanation for the increase in morphine production. The production quota for morphine is 10.55% higher than last year's quota and the highest amount since 2021.
One likely reason for the DEA’s decision is that the FDA recently added morphine tablets and injectable morphine solutions to its shortage list, due to discontinuations and short supplies. The ASHP has listed morphine in shortage for several months.
Morphine solutions and other injectable opioids are an important resource in hospitals, emergency rooms and surgery centers, where they are used in post-op care, sedation and anesthesia.
Morphine tablets are most often used to treat severe chronic pain.
“I fear there will be continued shortages resulting in many patients suffering from the DEA’s quota decisions.”
“In 2025, there were major shortages of morphine immediate release (15-mg, 30-mg tablets) and morphine extended release (mostly 30-mg tablets) that lasted 3-4 months and were disruptive to care. I mentioned morphine in my personal, submitted comments (to the Federal Register),” said Chad Kollas, MD, a palliative care physician in Florida.
“I suspect that others also complained about last year’s morphine shortages, which may have led to the increase in production of morphine in 2026. It is also the cheapest of the traditional opioids, which may have played a role in the decision. I’m disappointed that they held the line on the oxycodone reduction.”
“I don't know why the DEA would reduce oxycodone while increasing the morphine quota. It seems illogical since there are reports that both are in shortage at the clinical level,” says Lynn Webster, MD, a pain management expert and former president of the American Academy of Pain Medicine. “I fear there will be continued shortages resulting in many patients suffering from the DEAs quota decisions.
“They know patients are struggling to get access to both medications but they may think oxycodone is more likely to be abused than morphine. It appears they are trying to tell providers what they should prescribe. Yet they are not supposed to be involved in determining how medicine is practiced. Whether intentional or not, that is exactly what they are doing.”
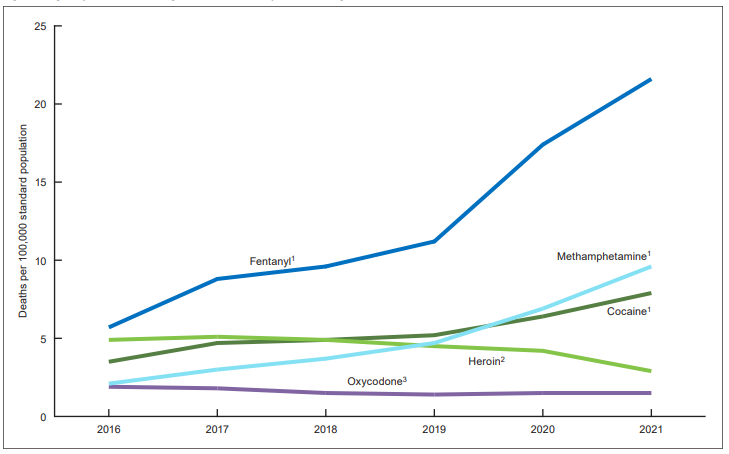
Even with this year’s increase in morphine production, DEA has reduced the supply of morphine by over 63% since 2015. Steep declines have also been made in quotas for hydrocodone (-73%), oxycodone (-71%), and codeine (-70%) over the past decade.
The DEA began cutting the opioid supply in response to pressure from Congress and anti-opioid activists, who claimed that prescription opioids were responsible for soaring overdose rates. While that claim has been largely debunked, opioid prescribing has continued to fall, as doctors became fearful of being accused of “overprescribing.”
The DEA says the “medical usage” of opioids fell by 10.5% in 2024 alone. The agency expects that trend to continue, while dismissing claims that its shrinking opioid production quotas have interfered with the practice of medicine.
“DEA's regulations do not impose restrictions on the amount and the type of medication that licensed practitioners can prescribe. DEA has consistently emphasized and supported the authority of individual practitioners under the CSA to administer, dispense, and prescribe controlled substances for the legitimate treatment of pain within acceptable medical standards,” DEA said.