Wear, Tear & Care: The Biomat
/By Jennifer Kain Kilgore, Columnist
Some pain relief modalities are unusual to the point that they’re out in the stratosphere. It’s also true that some products only work for some people. Just because a device doesn’t offer visible results the first, second, or even third time doesn’t mean it isn’t working.
That is why I have to keep an open mind and not make snap judgments based on concepts, websites, or promotional material.
Like, for instance, today’s topic: thermotherapy and the Amethyst Richway Biomat.
Amethysts?
Yes, amethysts -- February’s birthstone -- can also be beneficial in thermotherapy.
When speaking specifically about the Biomat, I should warn you that Richway’s website isn’t slick. The idea of amethysts being associated with anything health-related is out of most people’s comfort zone.
But hey, I’ve used the Biomat for upward of five years and fall onto it whenever I have sore muscles, which is constantly. It’s such a fixture in my life that at first I didn’t even think to discuss it. So here we go!
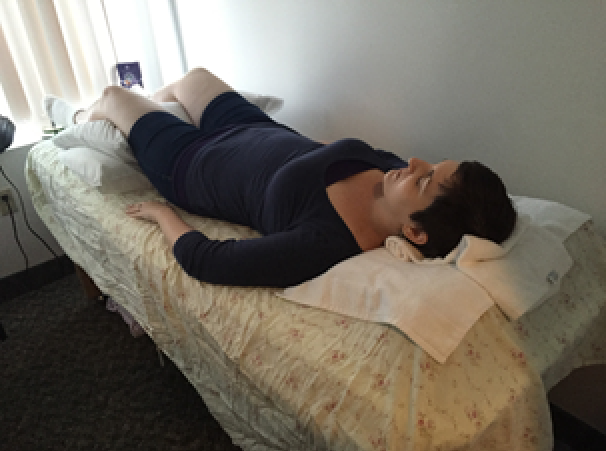
The Biomat. Behold:
This version is the full-body mat covered with a sheet. Underneath my head is the Biomat pillow. Each session can last for five minutes or twelve hours, depending on how much time you have available. The heat can reach temperatures of 158 F° degrees. Read on to find out why that number means absolutely nothing when it comes to treating pain.
The FDA has approved the marketing of the Biomat for a whole host of things: relaxation of muscles, improvement of circulation, temporary relief of muscle pain and/or spasms, and much more. There are specific range settings for certain medical conditions, though it is generally safe.
The science involved came to being when Drs. Erwin Neher and Bert Sakmann discovered how ions flow in and out of cells, which they called the “ion channel theory.” The two scientists revolutionized the field of cell biology and won the Nobel Prize in 1991 for their shared research.
If you’re like me, you have no idea what this means. However, this ion channel theory is put into play by the Biomat’s use of negative ions, which is then complemented by far infrared spectrum therapy (or thermotherapy, like what is found in saunas) and the amethysts embedded in the outer layer of the mat. These stones have been used for thousands of years for everything from fighting the evils of drunkenness to helping with meditation.
In modern times, researchers discovered that amethysts can carry an electrical charge. (Readers, are you still with me? Hang on, we’re almost there!) So, the infrared rays pass through the amethyst layer of the mat and then become “long wavelengths capable of safely penetrating the body as deeply as seven inches.” This heats up your core body temperature, encouraging your body to detoxify.
To put all of this in English: The Biomat creates an environment in which the patient can safely enjoy negative ion therapy and infrared therapy.
What does this mean for the person actually flopped onto the mat? It means a yummy, delicious, low-grade heat. And low-grade does mean low-grade, even if it can reach 158 F° degrees.
One time, my husband wanted to use a heating pad on a strained muscle and cranked it all the way up to eleven: “Honey, I don’t think this is working. I’ve maxed it out and it’s still not warm enough.”
That’s because it doesn’t generate heat the same way a traditional heating pad does. It gets toasty, sure, but you couldn’t cook meat on top of it. At its price point, you certainly wouldn’t want to cook any sort of food on it.
I mean, look at the controls. It’s like the cockpit of an airplane.
For me, the Biomat doesn’t present a dramatic “Before and After” picture. It’s not like Tiny Tim could throw away his crutches after using this product. I can’t think of things I couldn’t do before that, with the Biomat, I can do afterward. Even so, I would never stop using it.
This product is just better than a heating pad. When you hurt all over, you want something that reaches all over. Those of us with chronic pain, we use a plethora of heating devices and creams and patches in order to soothe sore muscles. The Biomat, while extremely expensive, is a full-body restoration and relaxation device. Even the mini mat fits into a chair and covers a lot of real estate.
At the end of a long day, I look forward to sitting on the Biomat. I sleep better when I use it; in the summer, for instance, even a low-grade heat is too much for me, and I go to bed feeling stiffer and more rickety, like a broken marionette. Those are the days I truly notice a difference. Studies have been conducted regarding thermotherapy and resulted in pain decreasing significantly (concurrently with anger and depression). It has even been used to treat cancer.
So does one recline upon the Biomat and come forth as a new person? No, not after one session, two sessions, twelve sessions, or fifty sessions, but you absolutely do feel better. Your muscles are soothed. Your knots unwind, even just a little bit. Your pain is quieted for a time. And that’s enough for me.
J. W. Kain is an attorney in the Greater Boston area who also works as a writer and editor in her spare time. She has chronic back and neck pain after two car accidents.
You can read more about J.W. on her blog, Wear, Tear, & Care.
The information in this column should not be considered as professional medical advice, diagnosis or treatment. It is for informational purposes only and represents the author’s opinions alone. It does not inherently express or reflect the views, opinions and/or positions of Pain News Network.