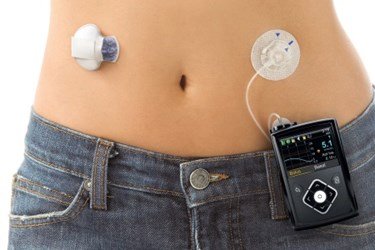
Medtronic is facing more than 60 lawsuits filed by injured patients and their families and the company believes it may be hit with claims for damages from thousands more patients, the company disclosed in an August Securities and Exchange Commission filing.
Medtronic pumps that allegedly dispensed too much, or too little, insulin have been blamed for contributing to at least a dozen patient deaths, according to lawsuits filed since 2019. Some cases have been settled under confidential terms, while others are pending or have been dismissed. Medtronic has denied any responsibility in response to the lawsuits.
In one pending case, a Las Vegas man using the pump allegedly fell into an “insulin-induced coma” that led to his death in 2020. In another 2020 case, a 67-year-old New Jersey resident collapsed at her home, dying later the same day at a local hospital.
The recall notice Medtronic sent to a 43-year-old Missouri man’s home arrived a few days after police found him dead on his bedroom floor, his family alleged in a lawsuit filed in August. “Simply too little, too late,” the suit reads. The case is pending, and Medtronic has yet to file an answer in court.
Medtronic declined to answer written questions from KFF Health News about the pumps and court cases. In an emailed statement, the company said it replaced pump rings with new ones “redesigned to reduce the risk of damage” and “fulfilled all pump replacement requests at no cost to customers.”
In April, Medtronic announced that the FDA had lifted the warning letter a few days after it approved a new version of the MiniMed pump system.
Shortcut to Market
The 1976 federal law that mandated safety testing for high-risk medical devices also created a far easier — and less costly — pathway to the marketplace. This process, known as a 510(k) clearance, requires manufacturers to show a new device they plan to sell has “substantial equivalence” to one already on the market, even if the prior product has been recalled.
Critics have worried for years that the 510(k)-approval scenario is too industry-friendly to protect patients from harm.
In July 2011, an Institute of Medicine report concluded that 510(k) was “not intended to evaluate the safety and effectiveness of medical devices” and said “a move away from the 510(k) clearance process should occur as soon as reasonably possible.”
More than a decade later, that hasn’t happened, even amid mounting controversy over the clearance of hundreds of devices that employ artificial intelligence.
The FDA now clears about 3,000 low- to moderate-risk devices every year through 510(k) review, which costs the device maker a standard FDA fee of about $22,000. That compares with about 30 approvals a year through the stricter premarketing requirements, which cost nearly $500,000 per device, according to FDA data.
Diana Zuckerman, president of the National Center for Health Research, said even many doctors don’t realize devices cleared for sale typically have not undergone clinical trials to establish their safety.
“Doctors are shocked to learn this,” she said. “Patients aren’t going to know it when their doctors don’t.”
In response to written questions from KFF Health News, the FDA said it “continues to believe in the merits of the 510(k) program and will continue to work to identify program improvements that strengthen the safety and effectiveness of 510(k) cleared devices.”
The FDA keeps a tight lid on data showing which devices manufacturers choose to demonstrate substantial equivalence — what the agency refers to as “predicate” devices.
“We can’t get detailed data,” said Sandra Rothenberg, a researcher at the Rochester Institute of Technology. “It’s very hard for researchers to determine the basis on which substantial equivalence is being made and to analyze if there are problems.”
Rothenberg cited the history of “metal-on-metal” artificial hip implants, which under 510(k) spawned many new brands — along with a disastrous toll of patient injuries. The implants could release metal particles that damaged bone and led to premature removal and replacement, a painful operation. Just four of these hip devices have been the target of more than 25,000 lawsuits seeking damages, court records show.
In early 2016, the FDA issued an order requiring safety testing before approving new metal-on-metal hip devices.
Alarm Bells
Two former Medtronic sales executives in California argue in a whistleblower lawsuit that the 510(k) process can be abused. According to the whistleblowers, the FDA approved the Puritan Bennett 980, or PB 980, ventilator in 2014 based on the assertion it was substantially equivalent to the PB 840, an earlier mechanical ventilator long viewed as the workhorse of the industry.
Medtronic’s subsidiary company Covidien made its claim even though the device has completely different “guts” and operates using software and other “substantially different” mechanisms, according to the whistleblowers’ suit. In response, Medtronic said it “believes the allegations are without merit and has moved to dismiss the case.” The case is pending.
The whistleblowers argue the PB 980 ventilator was plagued by dangerous malfunctions for years before its recall in late 2021. One ventilator billowed smoke in an intensive care unit while the whistleblowers were told by one hospital that “the wheels for the ventilator cart may actually fall off the ventilator during transport,” according to the suit.
Batteries could die without warning, kicking off a scramble to keep patients alive; monitor screens froze up repeatedly or otherwise went on the blink; and, in several cases, alarm bells warning of a patient emergency rang continuously and could be quieted only by unplugging the unit from the wall socket and pulling out its batteries, according to the suit.
The December 2021 recall of the PB 980 cited a “manufacturing assembly error” that the company said may cause the ventilator to become “inoperable.”
Medtronic said in an email that the ventilator “has helped thousands of patients around the world,” including playing a “critical role in the global response to the COVID-19 pandemic.”
Late Warnings
The FDA operates a massive database, called MAUDE, to alert regulators and the public to emerging device dangers. The FDA requires manufacturers to advise the agency when they learn their device may have caused or contributed to a death or serious injury, or malfunctioned in a way that might recur and cause harm. These reports must be submitted within 30 days unless a special exemption is granted.
But FDA officials acknowledge that many serious adverse events go unreported — just how many is anybody’s guess.
Since 2010, the FDA has cited companies more than 5,000 times for not handling, reviewing, or investigating complaints properly, or for not reporting adverse events on time. For instance, the FDA cited an Ohio company that made electric beds and other devices more than 15 times for failing to properly scrutinize complaints or report adverse events, including the death of a patient who allegedly became trapped between a bedrail and mattress, agency records show.
In about 10% of reports, more than a year or two elapsed from when a death or serious injury occurred and when the FDA received the reports, a KFF Health News analysis found. That works out to nearly 60,000 delayed reports a year.
Experts and lawmakers say the FDA needs to find a way to detect safety problems quicker.
Sens. Chuck Grassley (R-Iowa) and Elizabeth Warren (D-Mass.) have tried for years to persuade the agency to add unique device identifiers to Medicare payment claim forms to help track products that fail. In an email statement to KFF Health News, Grassley called that a “commonsense step we can take up front to mitigate risk, improve certainty and save money later.”
The FDA said it is working to “strike the right balance between assuring safety and fostering device innovation and patient access.” Yet it noted: “Additional resources are required to establish a fully functioning active surveillance system for medical devices.”
For now, injured patients suing device companies often cite the volume of adverse event reports to MAUDE, or FDA citations for failing to report them, to bolster claims that the company knew about product malfunctions but failed to correct them.
In one case, a New York man is suing manufacturer Boston Scientific, claiming injuries from a device called the AMS 800 that is used to treat stress urinary incontinence.
Though Boston Scientific says on its website that 200,000 men have been treated successfully, the lawsuit argues complaints piled up in MAUDE year after year and no action was taken — by the company or by regulators.
The number of complaints filed soared from six in 2016 to 2,753 in 2019, according to the suit. By far, the largest category involved incontinence, the condition the device was supposed to fix, according to the suit. Boston Scientific did not respond to a request for comment. The company has filed a motion to dismiss the case, which is pending.
By the FDA’s own count, more than 57,000 of some 74,000 complaints Medtronic received about the MiniMed insulin pump’s retainer rings were reported to the agency. The FDA said the complaints “were part of the information that led to the compliance actions.” The agency said it “approved design and manufacturing changes to the retainer ring to correct this issue” and “has reviewed information confirming the effectiveness of the modification.”
“What is the threshold for the FDA to step in and do something?” said Mara Schwartz, who is a nurse, diabetes educator, and pump user. “How many deaths or adverse events does there have to be?”
In 2020, she sued Medtronic, alleging she suffered seizures when the pump mistakenly delivered an overdose of insulin. Medtronic denied her claims, and the case has since been settled under confidential terms.