Drug Tests Scare Off Some Chronic Pain Patients
/By Pat Anson, Editor
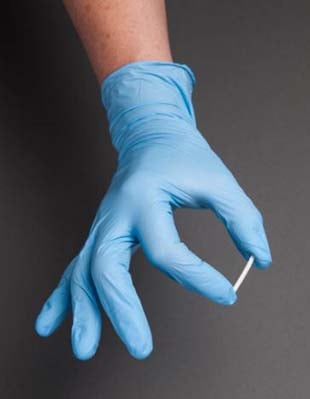
Urine drug testing has become standard protocol for many patients who are prescribed opioid pain medication. But a new study suggests the practice may be counterproductive, because it increases the odds a patient won’t come back for further treatment.
In a study involving 723 chronic pain patients being treated at a pain clinic, researchers at the University of Houston and the University of Texas Medical Branch at Galveston found that nearly a quarter (23.75%) who were given a urine drug screen on their first visit failed to show up for the next appointment.
The odds were even higher for those who tested positive for an illicit drug, but the “no show” trend also applied to patients whose drug tests were negative.
“Even those who tested negative for illicit substances in the UDS (urine drug screen) were more likely to be no-shows compared to those who did not get tested. This raises concerns that the UDS administered early in the doctor-patient relationship might have an inadvertent impact on injuring patient expectations of trust,” the researchers reported in the journal Pain Physician.
Only about 10% of those who weren’t tested skipped their follow-up appointment.
“It is a balancing act,” said Partha Krishnamurthy, director of the Institute for Health Care Marketing at the University of Houston’s Bauer College of Business. “On one hand, concerns about patient safety and public health necessitate the monitoring of patients on opioid medications. On the other hand, aggressive monitoring may interfere with the therapeutic alliance.”
Routine use of urine drug tests is one of the main recommendations in the CDC’s draft guidance for opioid prescribing, which calls for primary care physicians to “use urine drug testing before starting opioid therapy and consider urine drug testing at least annually.”
The scientific research behind that recommendation is considered weak, as is much of the evidence that standard “point of care” urine drug tests are reliable or accurate.
“I've only been saying that UDT (urine drug tests) harms patients and the patient/provider relationship for the past seven years and not a single physician, researcher, or healthcare provider of any kind supported my position. I guess common sense wasn't enough but now we have evidence,” said Mark Collen, an independent scholar and patient advocate.
“As I've stated previously in regards to UDT, the entire pain community will end up on the wrong side of history and it looks like that's beginning to occur.”
Researchers say one possible solution to the high-rate of patient “no shows” is for doctors to delay drug screening of new patients until they’ve had a chance to develop rapport and trust with them.
“Not testing is not an option,” the researchers said, while at the same time warning that routine testing may only make prescription drug abuse worse.
“If the patients are disengaging from the clinic, where are they going? Is the illicit market place their next stop? Thus, while UDS may induce the problematic patients to go away from the clinic, the problem of opioid misuse may continue to persist.”