“Guidelines for long-term opioid therapy should not be developed by the field of pain medicine alone. Rather, experts from general medicine, addiction medicine, and pain medicine should jointly reconsider how to increase the margin of safety,” they said.
That major overhaul came in 2016, when the Centers for Disease Control and Prevention released its controversial opioid guideline, which soon displaced all of the other guidelines. Chou was one of the co-authors of the CDC guideline – so it’s not altogether surprising that the AHRQ study reaffirms many of the CDC’s conclusions.
“Findings support the recommendation in the 2016 CDC guideline that opioids are not first-line therapy and to preferentially use nonopioid alternatives,” Chou and his colleagues wrote.
In preparing the AHRQ study, researchers sought input from a dozen outside experts, who served as technical experts and peer reviewers. Three of the 12 are PROP board members: Drs. Mark Sullivan and David Tauben were technical experts, and PROP vice-president Dr. Gary Franklin was a peer reviewer. Sullivan, Tauben and Franklin are all professors at the University of Washington, and played prominent roles in the development of Washington state’s opioid prescribing regulations, which are some of the toughest in the nation.
Another peer reviewer was Dr. Erin Krebs, an associate professor at the University of Minnesota Medical School. Krebs was the lead author of a controversial 2018 study that found non-opioid pain relievers worked better than opioids in treating osteoarthritis pain. While some critics said the study was poorly designed and amounted to junk science, it drew praise from Chou.
"The fact that opioids did worse is really pretty astounding," Chou told the Chicago Tribune. "It calls into question our beliefs about the benefits of opioids."
In addition to her work as a researcher, Krebs also appeared in a lecture series on opioid prescribing funded by the Steve Rummler Hope Foundation, which lobbies against the use of opioids. The non-profit foundation is the fiscal sponsor of PROP, and Kolodny and Ballantyne both serve on its medical advisory committee.
If these intertwining connections are making your head spin, there’s more.
Core Expert Group
Ballantyne, Franklin and Krebs served on the “Core Expert Group” that advised the CDC when it drafted its opioid guideline, and Tauben was on the CDC’s peer review panel. Kolodny and yet another PROP board member, Dr. David Juurlink, were part of a “Stakeholder Review Group” that provided input to the CDC.
When PNN filed a request under the Freedom of Information Act (FOIA) to see what kind of advice the Core Expert Group gave to the CDC, we were stiff-armed by the agency. The CDC sent us nearly 1,500 pages of documents, but most were so heavily redacted they were completely blank.
Even financial conflict of interest statements were scrubbed of information, with the CDC citing “personal privacy” and “deliberative process privilege” as reasons not to provide them in full.
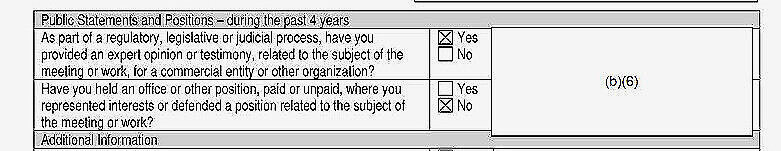
At least two unidentified members of the Core Expert Group worked for or consulted with organizations with an interest in opioids or other controlled substances. One of those individuals also provided “expert opinion or testimony,” which has become a lucrative sideline for some PROP members.
Critics wonder why federal health agencies keep bringing in consultants with obvious biases and conflicts of interest.
“The AHRQ review presents itself as an objective scientific analysis of the medical literature. In my opinion, the document is arguably contaminated with a political agenda,” says Dr. Dan Laird, a physician attorney in Las Vegas. “Some of those involved in the review could be perceived by the chronic pain community as having strong anti-opioid political views and biased ideas about the meaning and treatment of chronic pain.
“Most importantly, there is no input whatsoever from the chronic pain community in the review. There are certainly chronic pain patients with academic credentials that would qualify them to conduct a literature review. Several highly regarded academic physicians and scientists, known for opioid moderatism, are conspicuously absent as investigators, peer reviewers, or technical experts; these include pain medicine academicians such as Drs. Michael Schatman, Sean Mackey, Stefan Kertesz, and Vanila Singh.”
AHRQ Conflict Policy
If a peer reviewer or technical expert has a financial or professional conflict of interest, that does not automatically disqualify them in the eyes of the AHRQ, which will retain them “because of their unique clinical or content expertise.”
It’s also been a long-standing AHRQ policy not to disclose the names of advisors or authors until its reports are finalized.
“This policy is aimed at helping the authors maintain their independence by not being subject to lobbying by industry reps or others with conflicts of interest, either financial or intellectual,” AHRQ spokesman Bruce Seeman explained in an email.
But others wonder if the policy damages the agency’s reputation and the credibility of its research, by not giving the public a chance to review and comment on possible biases before a final report is released. The American Medical Association urged the AHRQ to change its policy last year.
“We would suggest that AHRQ publish the list of all those involved in any aspect of the report during the comment period to help remove any perception of potential conflict,” Dr. James Madara, the AMA’s Executive Director and CEO, wrote in a letter to the agency last year.
CDC Guideline Update
It might be tempting to dismiss the work of an obscure federal agency that produces wonky reports that are mostly read by public health researchers and government bureaucrats. That would be a mistake. It was a 2014 AHRQ report on opioids – co-authored by Chou – that played a foundational role in the CDC guideline.
Although the CDC guideline is voluntary and only intended for primary care physicians treating chronic pain, it has become mandatory policy for doctors in all specialties, as well as other federal agencies, dozens of states, insurers, pharmacy chains and law enforcement agencies. In effect, the guideline has delivered on the goals sought by Kolodny, Von Korff and Chou in 2011. The standard of care in pain management is no longer determined by pain specialists.
Chou and his colleagues hope the new AHRQ report will have a similar impact, not just in government, but throughout the healthcare system.
“The information in this report is intended to help healthcare decision makers — patients and clinicians, health system leaders, and policymakers, among others — make well-informed decisions and thereby improve the quality of healthcare services,” they said.
In addition to its report on opioid treatments for chronic pain, AHRQ has also finalized studies on the effectiveness of Nonopioid Medications for Chronic Pain and Nonpharmacologic Treatments for Chronic Pain, such as acupuncture and meditation.
All three reports will be utilized by the CDC as it prepares an update and expansion of its opioid guideline, which is expected in late 2021. That effort is being overseen by the Board of Scientific Counselors at the CDC’s Center for Injury Prevention & Control. Roger Chou happens to be one of its members.