BioWave Device Helps Vietnam Vet with Chronic Pain
/By Pat Anson, Editor
A few months ago, Vietnam veteran Gregg Gaston was depressed and suicidal. Gaston shared his story with PNN readers in a guest column, telling how he suffered from years of chronic pain caused by a failed back surgery, peripheral neuropathy and post-traumatic stress syndrome (PTSD).
Despite his pain, Gaston’s doctor told him he was being cut back to a single dose of tramadol, a mild opioid analgesic. That was the last straw for Gaston, who at the age of 62 was fed up with debilitating pain and doctors who no longer wanted to treat it with opioids. In protest, Gaston fired his doctor and refused to fill his last prescription for tramadol.
GREGG GASTON
“I've given up and am waiting now to die. I've lived a great life and have no expectations of my quality of life improving,” Gaston wrote. “Common sense is fast disappearing. I'm done fighting.”
Fast forward three months and there’s been a remarkable change in Gaston’s mood and quality of life. The folks at BioWave, a Connecticut medical device company, saw Gaston’s column and sent him one of their neurostimulation units, which use high frequency electrical impulses to block pain signals.
“I was skeptical at first. I really was,” Gaston says. “Being at the breaking point and feeling desperate, I was only too eager to try it. And for me, it really works. I’m not 100 percent pain free, but I can get out of bed in the morning. It’s great, it really is.”
Before he started using BioWave, Gaston says his pain level was usually a 7 or 8 on the pain scale. Today, even on a bad day, it’s only a 3.
“If you have chronic pain you know what a difference that is,” he says. “I used to often sleep no more than 2 hours at a time, now I often sleep through the entire night.”
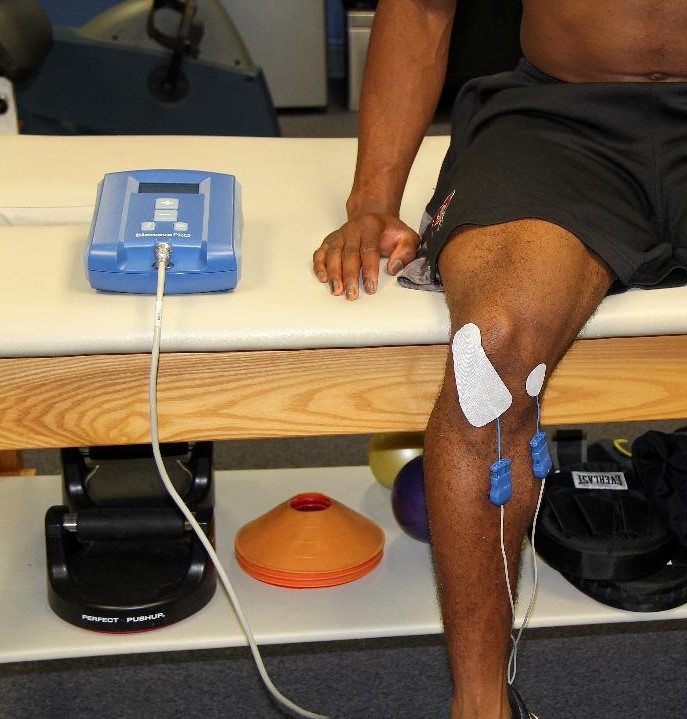
Think of BioWave as a more advanced version of a TENS unit. Electrodes wired to a battery and control unit send two high frequency signals through the skin into deep tissue, where they stimulate blood flow and block pain signals.
Each treatment takes 30 minutes, and the pain relief can last anywhere from a few hours to a few days, depending on the patient and their condition.
“I started using it several times a day. But as I got feeling better in my back, I dropped it down to once a day. Now it’s once every two or three days,” says Gaston, who takes no pain medication outside of an aspirin. “They saved my life. My quality of life is still not great, but it’s better than what it was.”
BIOWAVE IMAGE
BioWave has been around for a few years but is not widely known. It was first used by professional sports teams to treat athletes with sprains, tendonitis, muscle pain and other injuries. When it proved effective in treating chronic pain, dozens of pain clinics and VA hospitals started using BioWave devices.
BIOWAVE IMAGE
“Most of the treatments deal with chronic pain and I would say the majority deal with lumbar and cervical pain. That’s probably the bread and butter for our device, but certainly any extremity pain in the shoulder, elbow, elbow, wrist or ankle. We can really treat almost any location on the body,” says Brad Siff, BioWave’s founder and president.
The company says over 75% of patients respond to BioWave treatment, with a significant improvement in their pain scores, mobility and stiffness. The device can even help patients with complex conditions such as arachnoiditis, a chronic and incurable spinal disease.
“There’s a handful of anecdotal data that we have where arachnoiditis patients have responded. Similarly, patients with failed back surgery have been treated with BioWave and it helped them," Siff told PNN.
"I’m not saying it reduces their pain 100 percent, but some may get a 30, 40, 50 percent reduction in their pain and it lasts for a long period of time following the treatment."
BioWave is currently available only by prescription, but later this summer the company hopes to get FDA approval for a wearable over-the-counter home unit that can be purchased directly by patients. The final pricing hasn’t been determined, but Siff expects it to be between $300 to $400.
For more information, you can visit BioWave at their website by clicking here or by emailing them at info@biowave.com.