Stimulants Involved in Growing Number of Fentanyl Overdoses
/By Pat Anson, PNN Editor
The number of drug deaths involving both fentanyl and stimulants has soared in recent years, according to a new UCLA study that highlights the complex and changing nature of the U.S. overdose crisis.
Stimulants such as cocaine and methamphetamine are now involved in nearly a third of fentanyl-related overdoses, the most of any other drug class. Fentanyl is a synthetic opioid up to 100 times more potent than morphine and 50 times as potent as heroin.
In 2010, researchers say there were only 235 fatal overdoses in the U.S. involving illicit fentanyl and stimulants. In 2021, there were 34,429 drug deaths linked to fentanyl and stimulants, a 14,550% increase in a little over a decade.
"We're now seeing that the use of fentanyl together with stimulants is rapidly becoming the dominant force in the US overdose crisis," said lead author Joseph Friedman, PhD, an addiction researcher at the David Geffen School of Medicine at UCLA. "Fentanyl has ushered in a polysubstance overdose crisis, meaning that people are mixing fentanyl with other drugs, like stimulants, but also countless other synthetic substances. This poses many health risks and new challenges for health care providers.
“We have data and medical expertise about treating opioid use disorders, but comparatively little experience with the combination of opioids and stimulants together, or opioids mixed with other drugs. This makes it hard to stabilize people medically who are withdrawing from polysubstance use."
People who overdose on stimulants and other non-opioid substances mixed with fentanyl may not be as responsive to naloxone, which only works as an antidote to opioids.
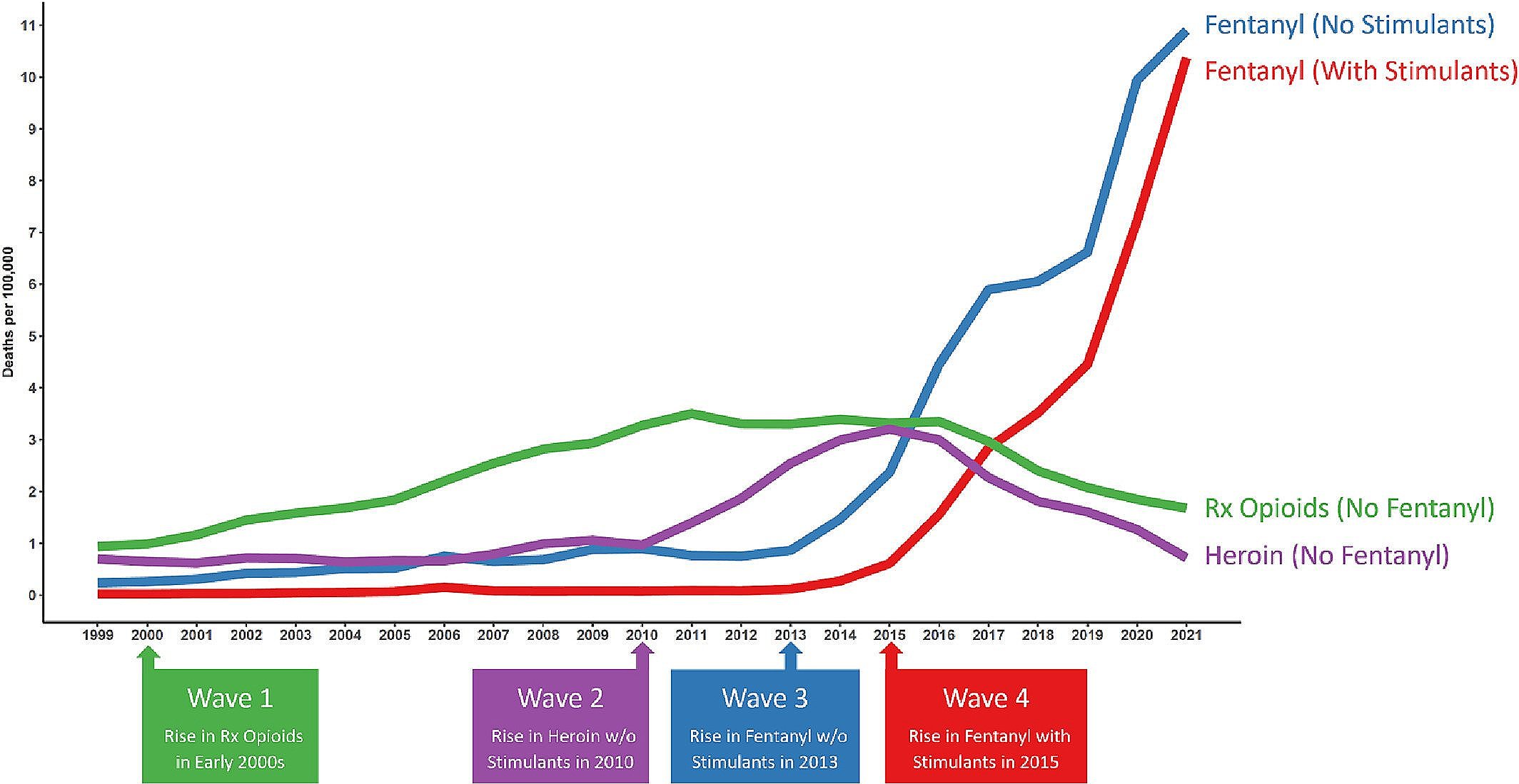
The study findings, published in the journal Addiction, highlight the four “waves” of the overdose crisis, which began with an increase in deaths from prescription opioids (Wave 1) in the early 2000s, followed by a rise in heroin deaths (Wave 2) in 2010, and fentanyl-related overdoses in 2013 (Wave 3). The fourth wave — overdoses from fentanyl and stimulants — began in 2015 and continues to escalate.
The Four Waves of Overdose Crisis
SOURCE: ADDICTION
Since cocaine, methamphetamine and other stimulants are not opioids, the findings undercut the long-held theory that the overdose crisis started with prescription opioids and is still being fueled by people addicted to them. Deaths involving prescription opioids and heroin have been in decline for several years.
Researchers found that fentanyl/stimulant deaths disproportionately affect African Americans and Native Americans. There are also geographical patterns to fentanyl/stimulant use. In the northeast US, fentanyl is usually combined with cocaine, while in the south and western US, fentanyl is most commonly found with methamphetamine.
"We suspect this pattern reflects the rising availability of, and preference for, low-cost, high-purity methamphetamine throughout the US, and the fact that the Northeast has a well-entrenched pattern of illicit cocaine use that has so far resisted the complete takeover by methamphetamine seen elsewhere in the country," Friedman said.
In addition to its low cost, drug users say methamphetamine helps prolong fentanyl’s “high” and delays the onset of withdrawal symptoms.
Counterfeit pills laced with fentanyl – which are frequently made to look like oxycodone or alprazolam (Xanax) – represent about a quarter of all illicit fentanyl seizures. Researchers say it is difficult to track deaths involving counterfeit pills because they are often mistaken for legitimate medication, so the data is not completely reliable.
In its most recent update on the overdose crisis, the CDC estimates there were a record 111,355 drug deaths in the 12-month period ending April 2023 -- about a thousand more deaths than the year before. Fentanyl and its analogues were involved in nearly 70% of the overdoses, stimulants were linked to about a third of them, and cocaine was involved in about a quarter of the drug deaths.