Is ‘Malicious Actor’ Behind Fentanyl Overdoses?
/By Pat Anson, Editor
The U.S. medical community is starting to pay more attention to the “burgeoning public health threat” posed by counterfeit prescription drugs made with illicit fentanyl.
And for the first time, public health researchers are suggesting that a “malicious actor” could be poisoning people intentionally with fake pills.
“The steep recent increase in overdose deaths and near-deaths nationwide involving fentanyl signals a new chapter in the epidemic of opioid use. Throughout the United States and Canada, seizures of pill presses, large quantities of active pharmaceutical ingredient in powder form, and counterfeit pills have been reported,” wrote Traci Green, PhD, Boston University School of Medicine, and Michael Gilbert, MPH, Epidemico Inc., in a research letter published in JAMA Internal Medicine.
“These highly potent pills could have been created by a malicious actor to intentionally poison consumers or attract the attention of law enforcement to redistributors.”
Green and Gilbert offered no evidence to support their theory, but said it was one of several "plausible hypotheses."
“I’m not going to really comment on speculation, because we deal in fact,” said DEA spokesman Rust Payne. “If you’re a drug trafficker, you don’t want to poison people. You want a regular customer base.”
Green and Gilbert were commenting on a case study also published in JAMA Internal Medicine that looked at 8 patients in the San Francisco area who were hospitalized late last year after ingesting fake alprazolam (Xanax) tablets that were later found to contain fentanyl. One victim was a baby boy just eight months old. All of the patients eventually recovered.
A few months later, 12 people died and dozens more were hospitalized in the Sacramento area after overdosing on fake Norco pills that were also made with fentanyl.
“This case series represents a burgeoning public health threat. Clinicians should be aware of the potential for further outbreaks and serious toxic effects associated with counterfeit prescription medications,” wrote lead author Ann Arens, MD, California Poison Control Center.
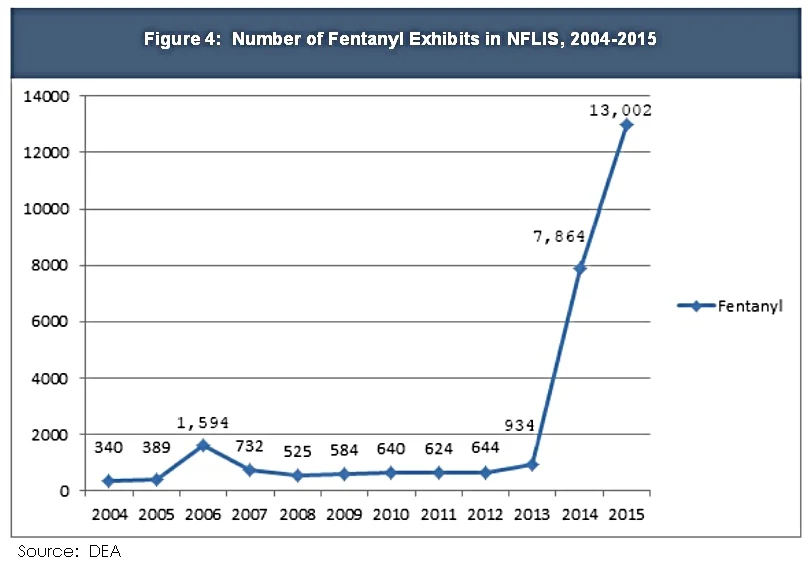
Fentanyl is a synthetic opioid 50 to 100 times more potent than morphine. It is prescribed legally in patches and lozenges to treat chronic pain, but illicitly manufactured fentanyl is fast becoming a scourge around the country. Since the fall of 2013, illicit fentanyl is blamed for over 5,000 deaths and the number of overdoses appears to be accelerating.
Illicit fentanyl was typically mixed with heroin to boost its potency but is now appearing in pill form, often disguised to look like pain medication such as Norco and oxycodone.
As Pain News Network has reported, the DEA recently issued a report saying the U.S. faced an unprecedented “fentanyl crisis” that was likely to grow worse. The agency blamed heavy consumer demand and the “enormous profit potential” of counterfeit medication.
But Green and Gilbert believe there could be a more sinister motive behind the overdoses, the “malicious actor” who wants to poison people.
“This hypothesis cannot be entirely ruled out by the evidence presented by Arens et al, but it is less likely because the quantity of fentanyl identified in the counterfeit alprazolam tablets was significantly greater than would be required to harm unwitting consumers,” they wrote.
The dangerous potency of the pills could also be accidental or the result of an inexperienced pill manufacturer, the two researchers said.
That is the more likely scenario, according to the DEA’s Payne, who says the pill press operations seized so far have been amateurish.
“If you’re just cutting fentanyl in a tablet in a lab somewhere, in someone’s garage or warehouse, you may be putting way too much fentanyl into a pill than anybody can withstand. And so that is what is going on right now,” Payne told PNN.
Regardless of the motive, Green and Gilbert called for an aggressive expansion in public health surveillance programs to detect new trends in drug use.
“New surveillance approaches and rapid expansion of evidence-based interventions are the missing parameters needed to shift the curve of the epidemic of opioid use,” they said.