Overdose Deaths Double for Teenagers Amid Fentanyl Surge
/By Pat Anson, PNN Editor
Drug deaths among U.S. teenagers have risen sharply in the last two years, according to a new study that found the number of fatal overdoses doubled for adolescents aged 14 to 18 years.
In 2019, there were 492 drug deaths among adolescents. In 2021, there were an estimated 1,146 fatal overdoses, a 133% increase.
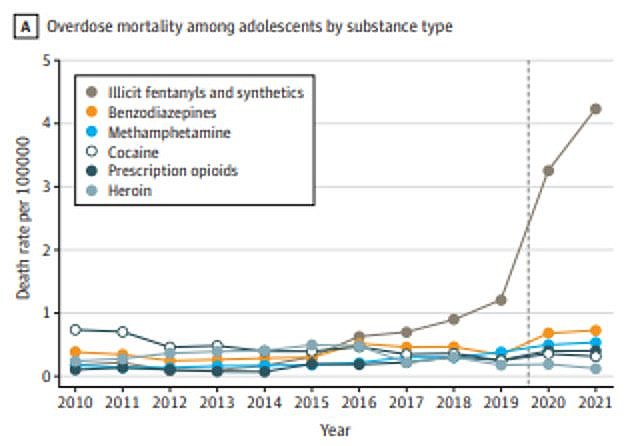
The vast majority of adolescent drug deaths last year involved illicit fentanyl (77%), followed by benzodiazepines (13%), methamphetamines (10%) and cocaine (7%). Less than 6% of the overdoses among teens involved a prescription opioid.
The study findings, reported in the journal JAMA, reflect what is happening in the overall U.S. population, with drug overdoses rising to record levels. They also mark the reversal of a decade long trend of fewer overdose deaths among teens, which coincided with declining rates of illicit drug use.
Researchers say adolescents may be unaware or naive about the risks posed by fentanyl, a synthetic opioid up to 100 times more potent than morphine and 50 times stronger than heroin. In a prescription, fentanyl plays a valuable role in treating severe pain, but as a street drug it can be deadly
“Beginning in 2020, adolescents experienced a greater relative increase in overdose mortality than the overall population, attributable in large part to fatalities involving fentanyls,” lead author Joseph Friedman, MPH, University of California, Los Angeles, reported in the journal JAMA.
“In the context of decreasing adolescent drug use rates nationally, these shifts suggest heightened risk from illicit fentanyls, which have variable and high potency. Since 2015, fentanyls have been increasingly added to counterfeit pills resembling prescription opioids, benzodiazepines, and other drugs, which adolescents may not identify as dangerous and which may be playing a key role in these shifts.”
U.S. Adolescent Overdose Deaths
SOURCE: jama
Friedman and his colleagues found the highest overdose rates among Native American, Alaska Native and Latino adolescents, reflecting what they called a “wider pattern of increasing racial and ethnic inequalities” in drug deaths.
Fentanyl is even killing kids who have not reached their teenage years. In California, a boy was recently arrested and charged with murder in the death of 12-year-old Dalilah Guerrero. The 16-year-old suspect allegedly sold a counterfeit pill made with fentanyl to the girl, who overdosed after crushing and snorting the tablet at a party in San Jose.
The spike in adolescent drug deaths comes even as substance abuse by teens fell to record lows. An annual survey by the University of Michigan found significant declines in all types of drug use by adolescents in 2021, with the use of prescription opioids falling to the lowest level in nearly two decades.
DEA Warns of Fentanyl Mass Overdoses
Public health experts and law enforcement agencies are growing increasingly alarmed by the rising number of fentanyl overdoses. Last week, the DEA warned of a nationwide spike in fentanyl-related mass overdose events, in which three or more overdoses occur in the same location.
In the past three months, at least seven mass overdoses were reported in Florida, Texas, Colorado, Nebraska, Missouri and Washington, DC, resulting in 29 deaths. Three people died in a hotel room in Cortez, Colorado after ingesting what that they thought were 30mg oxycodone pills, but were actually counterfeit pills containing fentanyl.
“Tragic events like these are being driven by fentanyl. Fentanyl is highly-addictive, found in all 50 states, and drug traffickers are increasingly mixing it with other types of drugs — in powder and pill form — in an effort to drive addiction and attract repeat buyers,” DEA Administrator Anne Milgram said in a letter to federal, state and local law enforcement agencies.
“We recommend that the members of your offices assume that all drugs encountered during enforcement activities now contain fentanyl. Given fentanyl’s extreme toxicity and the increases we are seeing in the distribution of polydrug substances containing fentanyl, please take all the precautions you would take when handling fentanyl whenever you interdict any illicit substance.”
A recent study by the National Institute of Drug Abuse estimated that over 9.6 million counterfeit pills containing fentanyl were seized by U.S. law enforcement agencies last year.